MolFinder: an evolutionary algorithm for the global optimization of molecular properties and the extensive exploration of chemical space using SMILES
- PMID: 33736687
- PMCID: PMC7977239
- DOI: 10.1186/s13321-021-00501-7
MolFinder: an evolutionary algorithm for the global optimization of molecular properties and the extensive exploration of chemical space using SMILES
Abstract
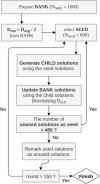
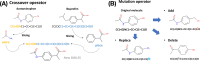
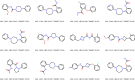
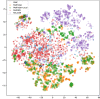
Here, we introduce a new molecule optimization method, MolFinder, based on an efficient global optimization algorithm, the conformational space annealing algorithm, and the SMILES representation. MolFinder finds diverse molecules with desired properties efficiently without any training and a large molecular database. Compared with recently proposed reinforcement-learning-based molecule optimization algorithms, MolFinder consistently outperforms in terms of both the optimization of a given target property and the generation of a set of diverse and novel molecules. The efficiency of MolFinder demonstrates that combinatorial optimization using the SMILES representation is a promising approach for molecule optimization, which has not been well investigated despite its simplicity. We believe that our results shed light on new possibilities for advances in molecule optimization methods.
Keywords: Chemical space; Evolutionary algorithm; Molecular optimization; SMILES.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
V-Dock: Fast Generation of Novel Drug-like Molecules Using Machine-Learning-Based Docking Score and Molecular Optimization.Int J Mol Sci. 2021 Oct 27;22(21):11635. doi: 10.3390/ijms222111635. Int J Mol Sci. 2021. PMID: 34769065 Free PMC article.
-
De Novo Molecule Design by Translating from Reduced Graphs to SMILES.J Chem Inf Model. 2019 Mar 25;59(3):1136-1146. doi: 10.1021/acs.jcim.8b00626. Epub 2018 Dec 21. J Chem Inf Model. 2019. PMID: 30525594
-
DrugEx v2: de novo design of drug molecules by Pareto-based multi-objective reinforcement learning in polypharmacology.J Cheminform. 2021 Nov 12;13(1):85. doi: 10.1186/s13321-021-00561-9. J Cheminform. 2021. PMID: 34772471 Free PMC article.
-
Hybridization of SMILES and chemical-environment-aware tokens to improve performance of molecular structure generation.Sci Rep. 2025 May 15;15(1):16892. doi: 10.1038/s41598-025-01890-7. Sci Rep. 2025. PMID: 40374848 Free PMC article.
-
Advances in Docking.Curr Med Chem. 2019;26(42):7555-7580. doi: 10.2174/0929867325666180904115000. Curr Med Chem. 2019. PMID: 30182836 Review.
Cited by
-
Molecular generation by Fast Assembly of (Deep)SMILES fragments.J Cheminform. 2021 Nov 14;13(1):88. doi: 10.1186/s13321-021-00566-4. J Cheminform. 2021. PMID: 34775976 Free PMC article.
-
Crossover operators for molecular graphs with an application to virtual drug screening.J Cheminform. 2025 Jun 17;17(1):97. doi: 10.1186/s13321-025-00958-w. J Cheminform. 2025. PMID: 40528251 Free PMC article.
-
Moldrug algorithm for an automated ligand binding site exploration by 3D aware molecular enumerations.J Cheminform. 2025 May 26;17(1):85. doi: 10.1186/s13321-025-01022-3. J Cheminform. 2025. PMID: 40420238 Free PMC article.
-
Generative Models as an Emerging Paradigm in the Chemical Sciences.J Am Chem Soc. 2023 Apr 26;145(16):8736-8750. doi: 10.1021/jacs.2c13467. Epub 2023 Apr 13. J Am Chem Soc. 2023. PMID: 37052978 Free PMC article. Review.
-
CMOMO: a deep multi-objective optimization framework for constrained molecular multi-property optimization.Brief Bioinform. 2025 Jul 2;26(4):bbaf335. doi: 10.1093/bib/bbaf335. Brief Bioinform. 2025. PMID: 40635191 Free PMC article.
References
-
- Kuhn C, Beratan DN. Inverse strategies for molecular design. J Phys Chem. 1996;100(25):10595–10599. doi: 10.1021/jp960518i. - DOI
-
- Schneider P, Walters WP, Plowright AT, Sieroka N, Listgarten J, Goodnow RA, Fisher J, Jansen JM, Duca JS, Rush TS, Zentgraf M, Hill JE, Krutoholow E, Kohler M, Blaney J, Funatsu K, Luebkemann C, Schneider G. Rethinking drug design in the artificial intelligence era. Nat Rev Drug Discov. 2020;19(5):353–364. doi: 10.1038/s41573-019-0050-3. - DOI - PubMed
-
- Elton DC, Boukouvalas Z, Fuge MD, Chung PW. Deep learning for molecular design—a review of the state of the art. Mol Syst Des Eng. 2019;4(4):828–849. doi: 10.1039/c9me00039a. - DOI
-
- Weininger D. SMILES, a chemical language and information system: 1: introduction to methodology and encoding rules. J Chem Inf Comput Sci. 1988;28(1):31–36. doi: 10.1021/ci00057a005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

