GPR18 drives FAAH inhibition-induced neuroprotection against HIV-1 Tat-induced neurodegeneration
- PMID: 33736974
- PMCID: PMC8984429
- DOI: 10.1016/j.expneurol.2021.113699
GPR18 drives FAAH inhibition-induced neuroprotection against HIV-1 Tat-induced neurodegeneration
Abstract
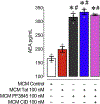
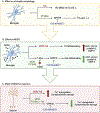
Human immunodeficiency virus type 1 (HIV-1) is known to provoke microglial immune responses which likely play a paramount role in the development of chronic neuroinflammatory conditions and neuronal damage related to HIV-1 associated neurocognitive disorders (HAND). In particular, HIV-1 Tat protein is a proinflammatory neurotoxin which predisposes neurons to synaptodendritic injury. Drugs targeting the degradative enzymes of endogenous cannabinoids have shown promise in reducing inflammation with minimal side effects in rodent models. Considering that markers of neuroinflammation can predict the extent of neuronal injury in HAND patients, we evaluated the neurotoxic effect of HIV-1 Tat-exposed microglia following blockade of fatty acid amid hydrolyze (FAAH), a catabolic enzyme responsible for degradation of endocannabinoids, e.g. anandamide (AEA). In the present study, cultured murine microglia were incubated with Tat and/or a FAAH inhibitor (PF3845). After 24 h, cells were imaged for morphological analysis and microglial conditioned media (MCM) was collected. Frontal cortex neuron cultures (DIV 7-11) were then exposed to MCM, and neurotoxicity was assessed via live cell calcium imaging and staining of actin positive dendritic structures. Results demonstrate a strong attenuation of microglial responses to Tat by PF3845 pretreatment, which is indicated by 1) microglial changes in morphology to a less proinflammatory phenotype using fractal analysis, 2) a decrease in release of neurotoxic cytokines/chemokines (MCP-1/CCL2) and matrix metalloproteinases (MMPs; MMP-9) using ELISA/multiplex assays, and 3) enhanced production of endocannabinoids (AEA) using LC/MS/MS. Additionally, PF3845's effects on Tat-induced microglial-mediated neurotoxicity, decreased dysregulation of neuronal intracellular calcium and prevented the loss of actin-positive staining and punctate structure in frontal cortex neuron cultures. Interestingly, these observed neuroprotective effects appeared to be independent of cannabinoid receptor activity (CB1R & CB2R). We found that a purported GPR18 antagonist, CID-85469571, blocked the neuroprotective effects of PF3845 in all experiments. Collectively, these experiments increase understanding of the role of FAAH inhibition and Tat in mediating microglial neurotoxicity in the HAND condition.
Keywords: Anandamide; Endocannabinoid; Fatty acid amide hydrolase; G protein coupled receptor; HIV-1 Tat; Matrix metalloproteinase; Microglia; Monocyte chemoattractant protein 1; Neuroinflammation; Neuroprotection.
Published by Elsevier Inc.
Figures

Similar articles
-
Neuroprotective effects of fatty acid amide hydrolase catabolic enzyme inhibition in a HIV-1 Tat model of neuroAIDS.Neuropharmacology. 2018 Oct;141:55-65. doi: 10.1016/j.neuropharm.2018.08.013. Epub 2018 Aug 13. Neuropharmacology. 2018. PMID: 30114402 Free PMC article.
-
Inhibitory Neurotransmission Is Sex-Dependently Affected by Tat Expression in Transgenic Mice and Suppressed by the Fatty Acid Amide Hydrolase Enzyme Inhibitor PF3845 via Cannabinoid Type-1 Receptor Mechanisms.Cells. 2022 Mar 2;11(5):857. doi: 10.3390/cells11050857. Cells. 2022. PMID: 35269478 Free PMC article.
-
Endocannabinoids exert CB1 receptor-mediated neuroprotective effects in models of neuronal damage induced by HIV-1 Tat protein.Mol Cell Neurosci. 2017 Sep;83:92-102. doi: 10.1016/j.mcn.2017.07.003. Epub 2017 Jul 19. Mol Cell Neurosci. 2017. PMID: 28733129 Free PMC article.
-
Mini-review: The therapeutic role of cannabinoids in neuroHIV.Neurosci Lett. 2021 Apr 17;750:135717. doi: 10.1016/j.neulet.2021.135717. Epub 2021 Feb 12. Neurosci Lett. 2021. PMID: 33587986 Free PMC article. Review.
-
Transactivator of Transcription (Tat)-Induced Neuroinflammation as a Key Pathway in Neuronal Dysfunction: A Scoping Review.Mol Neurobiol. 2024 Nov;61(11):9320-9346. doi: 10.1007/s12035-024-04173-w. Epub 2024 Apr 17. Mol Neurobiol. 2024. PMID: 38627350 Free PMC article.
Cited by
-
Possible Association of Nucleobindin-1 Protein with Depressive Disorder in Patients with HIV Infection.Brain Sci. 2022 Aug 29;12(9):1151. doi: 10.3390/brainsci12091151. Brain Sci. 2022. PMID: 36138887 Free PMC article.
-
Deciphering Complex Interactions in Bioactive Lipid Signaling.Molecules. 2023 Mar 14;28(6):2622. doi: 10.3390/molecules28062622. Molecules. 2023. PMID: 36985594 Free PMC article.
-
Genetic Variants of Fatty Acid Amide Hydrolase Modulate Acute Inflammatory Responses to Colitis in Adult Male Mice.Front Cell Neurosci. 2021 Nov 22;15:764706. doi: 10.3389/fncel.2021.764706. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34916909 Free PMC article.
-
Monoacylglycerol Lipase Inhibitor MJN110 Reduces Neuronal Hyperexcitability, Restores Dendritic Arborization Complexity, and Regulates Reward-Related Behavior in Presence of HIV-1 Tat.Front Neurol. 2021 Aug 16;12:651272. doi: 10.3389/fneur.2021.651272. eCollection 2021. Front Neurol. 2021. PMID: 34484091 Free PMC article.
-
On the Role of Paraoxonase-1 and Chemokine Ligand 2 (C-C motif) in Metabolic Alterations Linked to Inflammation and Disease. A 2021 Update.Biomolecules. 2021 Jul 1;11(7):971. doi: 10.3390/biom11070971. Biomolecules. 2021. PMID: 34356595 Free PMC article. Review.
References
-
- Ben Haij N, Planès R, Leghmari K, Serrero M, Delobel P, Izopet J, BenMohamed L, Bahraoui E, 2015. HIV-1 tat protein induces production of proinflammatory cytokines by human dendritic cells and monocytes/macrophages through engagement of TLR4-MD2-CD14 complex and activation of NF-κB pathway. PLoS One 10, e0129425. - PMC - PubMed
-
- Block ML, Zecca L, Hong J-S, 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci 8, 57–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

