Structural and molecular perspectives of SARS-CoV-2
- PMID: 33737214
- PMCID: PMC7959701
- DOI: 10.1016/j.ymeth.2021.03.007
Structural and molecular perspectives of SARS-CoV-2
Abstract
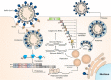
Recent emergence of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transpired into pandemic coronavirus disease 2019 (COVID-19). SARS-CoV-2 has been rapidly transmitted across the globe within a short period of time, with more than 106 million cases and 2.3 million deaths. The continuous rise in worldwide cases of COVID-19, transmission dynamics of SARS-CoV-2 including re-infections and enormous case-fatality rates emphasizes the urgent need of potential preventive and therapeutic measures. The development of effective therapeutic and preventive measures relies on understanding the molecular and cellular mechanism of replication exhibited by SARS-CoV-2. The structure of SARS-CoV-2 is ranging from 90-120 nm that comprises surface viral proteins including spike, envelope, membrane which are attached in host lipid bilayer containing the helical nucleocapsid comprising viral RNA. Spike (S) glycoprotein initiates the attachment of SARS-CoV-2 with a widely expressed cellular receptor angiotensin-converting enzyme 2 (ACE2), and subsequent S glycoprotein priming via serine protease TMPRSS2. Prominently, comprehensive analysis of structural insights into the crucial SARS-CoV-2 proteins may lead us to design effective therapeutics molecules. The present article, emphasizes the molecular and structural perspective of SARS-CoV-2 including mechanistic insights in its replication.
Keywords: ACE2; COVID-19; Mpro; Nucleocapsid; SARS-CoV-2; Spike-glycoprotein; nsp10/nsp16 2′-O-methylase.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interest. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. We have not received any specific funding for this work. SK Saxena is supported by CCRH, Government of India. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


Similar articles
-
Structural basis of severe acute respiratory syndrome coronavirus 2 infection.Curr Opin HIV AIDS. 2021 Jan;16(1):74-81. doi: 10.1097/COH.0000000000000658. Curr Opin HIV AIDS. 2021. PMID: 33186231 Review.
-
In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs.J Med Virol. 2020 Oct;92(10):2105-2113. doi: 10.1002/jmv.25987. Epub 2020 May 17. J Med Virol. 2020. PMID: 32383269 Free PMC article.
-
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor.Cell. 2020 Apr 16;181(2):271-280.e8. doi: 10.1016/j.cell.2020.02.052. Epub 2020 Mar 5. Cell. 2020. PMID: 32142651 Free PMC article.
-
COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics.Infection. 2021 Apr;49(2):199-213. doi: 10.1007/s15010-020-01516-2. Epub 2020 Sep 4. Infection. 2021. PMID: 32886331 Free PMC article.
-
Potential therapeutic approaches for the early entry of SARS-CoV-2 by interrupting the interaction between the spike protein on SARS-CoV-2 and angiotensin-converting enzyme 2 (ACE2).Biochem Pharmacol. 2021 Oct;192:114724. doi: 10.1016/j.bcp.2021.114724. Epub 2021 Aug 8. Biochem Pharmacol. 2021. PMID: 34371003 Free PMC article. Review.
Cited by
-
Evolving trend change during the COVID-19 pandemic.Front Public Health. 2022 Sep 20;10:957265. doi: 10.3389/fpubh.2022.957265. eCollection 2022. Front Public Health. 2022. PMID: 36203708 Free PMC article.
-
Omicron - The new SARS-CoV-2 challenge?Rev Med Virol. 2022 Jul;32(4):e2358. doi: 10.1002/rmv.2358. Epub 2022 Apr 21. Rev Med Virol. 2022. PMID: 35445774 Free PMC article. Review.
-
Tracing the origin of Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A systematic review and narrative synthesis.J Med Virol. 2022 Dec;94(12):5766-5779. doi: 10.1002/jmv.28060. Epub 2022 Sep 7. J Med Virol. 2022. PMID: 35945190 Free PMC article.
-
Progress and Challenges Toward Generation and Maintenance of Long-Lived Memory T Lymphocyte Responses During COVID-19.Front Immunol. 2022 Feb 17;12:804808. doi: 10.3389/fimmu.2021.804808. eCollection 2021. Front Immunol. 2022. PMID: 35250966 Free PMC article. Review.
-
B-Cell Epitopes-Based Chimeric Protein from SARS-CoV-2 N and S Proteins Is Recognized by Specific Antibodies in Serum and Urine Samples from Patients.Viruses. 2023 Sep 5;15(9):1877. doi: 10.3390/v15091877. Viruses. 2023. PMID: 37766284 Free PMC article.
References
-
- Coronavirus disease (COVID-19) pandemic. World Health Organization (WHO). https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed on 02 Feb 2021).
-
- Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

