Murine myeloid cell MCPIP1 suppresses autoimmunity by regulating B-cell expansion and differentiation
- PMID: 33737335
- PMCID: PMC7988765
- DOI: 10.1242/dmm.047589
Murine myeloid cell MCPIP1 suppresses autoimmunity by regulating B-cell expansion and differentiation
Abstract
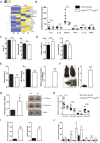
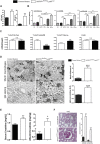
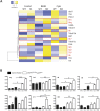
Myeloid-derived cells, in particular macrophages, are increasingly recognized as critical regulators of the balance of immunity and tolerance. However, whether they initiate autoimmune disease or perpetuate disease progression in terms of epiphenomena remains undefined.Here, we show that depletion of MCPIP1 in macrophages and granulocytes (Mcpip1fl/fl-LysMcre+ C57BL/6 mice) is sufficient to trigger severe autoimmune disease. This was evidenced by the expansion of B cells and plasma cells and spontaneous production of autoantibodies, including anti-dsDNA, anti-Smith and anti-histone antibodies. Consequently, we document evidence of severe skin inflammation, pneumonitis and histopathologic evidence of glomerular IgG deposits alongside mesangioproliferative nephritis in 6-month-old mice. These phenomena are related to systemic autoinflammation, which secondarily induces a set of cytokines such as Baff, Il5, Il9 and Cd40L, affecting adaptive immune responses. Therefore, abnormal macrophage activation is a key factor involved in the loss of immune tolerance.Overall, we demonstrate that deficiency of MCPIP1 solely in myeloid cells triggers systemic lupus-like autoimmunity and that the control of myeloid cell activation is a crucial checkpoint in the development of systemic autoimmunity.
Keywords: Autoantibodies; Autoimmunity; Lupus nephritis; MCPIP1; Myeloid cells.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Bengtsson, A. A., Sturfelt, G., Truedsson, L., Blomberg, J., Alm, G., Vallin, H. and Rönnblom, L. (2000). Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 9, 664-671. 10.1191/096120300674499064 - DOI - PubMed
-
- Birnberg, T., Bar-On, L., Sapoznikov, A., Caton, M. L., Cervantes-Barragán, L., Makia, D., Krauthgamer, R., Brenner, O., Ludewig, B., Brockschnieder, D.et al. (2008). Lack of conventional dendritic cells is compatible with normal development and T Cell homeostasis, but causes myeloid proliferative syndrome. Immunity 29, 986-997. 10.1016/j.immuni.2008.10.012 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

