Primary results from TAIL: a global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer
- PMID: 33737339
- PMCID: PMC7978274
- DOI: 10.1136/jitc-2020-001865
Primary results from TAIL: a global single-arm safety study of atezolizumab monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer
Abstract
Background: Atezolizumab treatment improves survival, with manageable safety, in patients with previously treated advanced/metastatic non-small cell lung cancer. The global phase III/IV study TAIL (NCT03285763) was conducted to evaluate the safety and efficacy of atezolizumab monotherapy in a clinically diverse population of patients with previously treated non-small cell lung cancer, including those not eligible for pivotal trials.
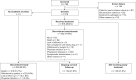
Methods: Patients with stage IIIB/IV non-small cell lung cancer whose disease progressed after 1-2 lines of chemotherapy were eligible for this open-label, single-arm, multicenter study, including those with severe renal impairment, an Eastern Cooperative Oncology Group performance status of 2, prior anti-programmed death 1 (PD-1) therapy, and autoimmune disease. Atezolizumab was administered intravenously (1200 mg every 3 weeks). Coprimary endpoints were treatment-related serious adverse events and immune-related adverse events.
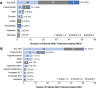
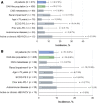
Results: 619 patients enrolled and 615 received atezolizumab. At data cutoff, the median follow-up was 12.6 months (95% CI 11.9 to 13.1). Treatment-related serious adverse events occurred in 7.8% and immune-related adverse events in 8.3% of all patients and as follows, respectively, in these subgroups: renal impairment (n=78), 11.5% and 12.8%; Eastern Cooperative Oncology Group performance status of 2 (n=61), 14.8% and 8.2%; prior anti-PD-1 therapy (n=39), 5.1% and 7.7%; and autoimmune disease (n=30), 6.7% and 10.0%. No new safety signals were reported. In the overall population, the median overall survival was 11.1 months (95% CI 8.9 to 12.9), the median progression-free survival was 2.7 months (95% CI 2.1 to 2.8) and the objective response rate was 11%.
Conclusions: This study confirmed the benefit-risk profile of atezolizumab monotherapy in a clinically diverse population of patients with previously treated non-small cell lung cancer. These safety and efficacy outcomes may inform treatment decisions for patients generally excluded from checkpoint inhibitor trials.
Keywords: PD-L1 inhibitor; checkpoint inhibitor; clinical trials; immunotherapy; lung neoplasms; metastatic; phase IIII clinical trial; subgroup analysis.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Support of the parent study and funding of editorial support were provided by F. Hoffmann-La Roche/Genentech. AA is a consultant to Merck Sharpe & Dohme, AstraZeneca, Bristol Myers Squibb, and F. Hoffmann-La Roche and has received research funding from Bristol Myers Squibb, F. Hoffmann-La Roche, and Celgene and honoraria from Eli Lilly and Pfizer. SA is a consultant to and has received research funding, honoraria, and travel expenses from F. Hoffmann-La Roche, Bristol Myers Squibb, and Celgene, is a consultant to Merck Sharpe & Dohme, and has received research funding, travel expenses, and honoraria from Novartis, Eli Lilly, and AstraZeneca. BRV is a consultant to Merck Sharpe & Dohme and Eli Lilly and has received honoraria and travel expenses from F. Hoffmann-La Roche, Eli Lilly, Bristol Myers Squibb and Merck Sharpe & Dohme. DR-A is an advisor to and has received speaker honoraria from Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck Sharpe & Dohme, F. Hoffmann-La Roche, Pfizer, and AstraZeneca. JA-A is a consultant to, on a speaker bureau for, and has received travel expenses from Merck Sharpe & Dohme, AstraZeneca, Pfizer, Boehringer Ingelheim, Eli Lilly, Novartis, F. Hoffmann-La Roche, and Bristol Myers Squibb. HJMS is a consultant to Merck Sharpe & Dohme, AstraZeneca, and Bristol Myers Squibb and is secretary of the oncology section of NVALT (Dutch lung physician’s organization) and president of the Dutch Lung Cancer Audit. JY has nothing to disclose. KS is on a speaker bureau for Merck Sharpe & Dohme, has received research funding from F. Hoffmann-La Roche and Novartis, has received research funding and travel expenses from Bristol Myers Squibb, and has received travel expenses from Genesis. KT, JT, and AC are employees of F. Hoffmann-La Roche. HP and PP-M are employees of Genentech. TN-D is a consultant to Amgen, Bayer, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck Sharpe & Dohme, Novartis, Otsuka, Pfizer, Roche, and Takeda, and is on a speaker bureau for AstraZeneca, Merck Sharpe & Dohme, F. Hoffmann-La Roche, and Takeda.
Figures
References
-
- NCCN Clinical Practice Guidelines in Oncology . Non-small cell lung cancer. Version 7.2019, 2019. Available: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf [Accessed 18 Oct 2019].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
