Mid-German Sepsis Cohort (MSC): a prospective observational study of sepsis survivorship
- PMID: 33737430
- PMCID: PMC7978081
- DOI: 10.1136/bmjopen-2020-043352
Mid-German Sepsis Cohort (MSC): a prospective observational study of sepsis survivorship
Abstract
Purpose: The Mid-German Sepsis Cohort (MSC) aims to investigate mid-term and long-term functional disabilities in sepsis survivors from intensive care unit (ICU) discharge until 1 year after. Secondary, post-acute mortality and morbidity, health-related quality of life and healthcare utilisation will be investigated.
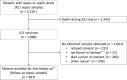
Participants: The MSC comprises adult (aged ≥18 years) patients who were treated for (severe) sepsis or septic shock on ICU. The participants were recruited between 15 April 2016 and 30 November 2018 from five German centres. Three thousand two hundred and ten patients with sepsis were identified, of which 1968 survived their ICU stay and were eligible for enrolment in the follow-up cohort. Informed consent for follow-up assessment was provided by 907 patients (46.1% of eligible patients).
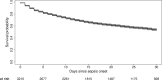
Findings to date: The recruitment of the participants for follow-up assessments and the baseline data collection is completed. Incidence of sepsis was 116.7 patients per 1000 ICU patients. In this cohort profile, we provide an overview of the demographics and the clinical characteristics of both the overall sepsis cohort and the ICU survivors who provided informed consent for follow-up assessment (907 out of 1968 ICU survivors (46.1%)).
Future plans: The follow-ups are conducted 3, 6 and 12 months after ICU discharge. Another yearly follow-up up to 5 years after ICU discharge is pursued. Several cooperation and satellite projects were initiated. This prospective cohort offers a unique resource for research on long-term sequelae of sepsis survivors.
Trial registration number: German Clinical Trials Registry (DRKS00010050).
Keywords: adult intensive & critical care; epidemiology; infectious diseases.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CSH declares funding from the German Federal Joint Committee Innovations-Funds and the European Society of Intensive Care Medicine. All other authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
