Noradrenergic Signaling Disengages Feedforward Transmission in the Nucleus Accumbens Shell
- PMID: 33737458
- PMCID: PMC8084318
- DOI: 10.1523/JNEUROSCI.2420-20.2021
Noradrenergic Signaling Disengages Feedforward Transmission in the Nucleus Accumbens Shell
Abstract
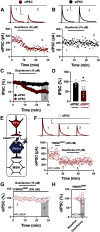
The nucleus accumbens shell (NAcSh) receives extensive monoaminergic input from multiple midbrain structures. However, little is known how norepinephrine (NE) modulates NAc circuit dynamics. Using a dynamic electrophysiological approach with optogenetics, pharmacology, and drugs acutely restricted by tethering (DART), we explored microcircuit-specific neuromodulatory mechanisms recruited by NE signaling in the NAcSh of parvalbumin (PV)-specific reporter mice. Surprisingly, NE had little direct effect on modulation of synaptic input at medium spiny projection neurons (MSNs). In contrast, we report that NE transmission selectively modulates glutamatergic synapses onto PV-expressing fast-spiking interneurons (PV-INs) by recruiting postsynaptically-localized α2-adrenergic receptors (ARs). The synaptic effects of α2-AR activity decrease PV-IN-dependent feedforward inhibition onto MSNs evoked via optogenetic stimulation of cortical afferents to the NAcSh. These findings provide insight into a new circuit motif in which NE has a privileged line of communication to tune feedforward inhibition in the NAcSh.SIGNIFICANCE STATEMENT The nucleus accumbens (NAc) directs reward-related motivational output by integrating glutamatergic input with diverse neuromodulatory input from monoamine centers. The present study reveals a synapse-specific regulatory mechanism recruited by norepinephrine (NE) signaling within parvalbumin-expressing interneuron (PV-IN) feedforward inhibitory microcircuits. PV-IN-mediated feedforward inhibition in the NAc is instrumental in coordinating NAc output by synchronizing the activity of medium spiny projection neurons (MSNs). By negatively regulating glutamatergic transmission onto PV-INs via α2-adrenergic receptors (ARs), NE diminishes feedforward inhibition onto MSNs to promote NAc output. These findings elucidate previously unknown microcircuit mechanisms recruited by the historically overlooked NE system in the NAc.
Keywords: adrenergic receptor; feedforward inhibition; neorepinephrine; nucleus accumbens; parvalbumin interneurons; prefrontal cortex.
Copyright © 2021 the authors.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
