Karonudib has potent anti-tumor effects in preclinical models of B-cell lymphoma
- PMID: 33737576
- PMCID: PMC7973795
- DOI: 10.1038/s41598-021-85613-8
Karonudib has potent anti-tumor effects in preclinical models of B-cell lymphoma
Abstract
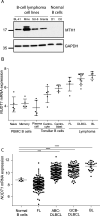
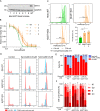
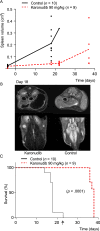
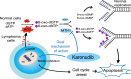
Chemo-immunotherapy has improved survival in B-cell lymphoma patients, but refractory/relapsed diseases still represent a major challenge, urging for development of new therapeutics. Karonudib (TH1579) was developed to inhibit MTH1, an enzyme preventing oxidized dNTP-incorporation in DNA. MTH1 is highly upregulated in tumor biopsies from patients with diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma, hence confirming a rationale for targeting MTH1. Here, we tested the efficacy of karonudib in vitro and in preclinical B-cell lymphoma models. Using a range of B-cell lymphoma cell lines, karonudib strongly reduced viability at concentrations well tolerated by activated normal B cells. In B-cell lymphoma cells, karonudib increased incorporation of 8-oxo-dGTP into DNA, and prominently induced prometaphase arrest and apoptosis due to failure in spindle assembly. MTH1 knockout cell lines were less sensitive to karonudib-induced apoptosis, but were displaying cell cycle arrest phenotype similar to the wild type cells, indicating a dual inhibitory role of the drug. Karonudib was highly potent as single agent in two different lymphoma xenograft models, including an ABC DLBCL patient derived xenograft, leading to prolonged survival and fully controlled tumor growth. Together, our preclinical findings provide a rationale for further clinical testing of karonudib in B-cell lymphoma.
Conflict of interest statement
A patent has been filed with TH588 and TH1579 where T.H. is listed as inventor. The Intellectual Property Right is owned by the non-profit Thomas Helleday Foundation for Medical Research (THF). T.H., U.W.B., K.S. and H.G. are board members of the THF. THF is sponsor for on-going clinical trial with TH1579. Oxcia AB is assisting THF in TH1579 clinical trial and U.W.B is chairman of Oxcia AB. H.G., U.W.B., T.P., K.S. and T.H. are shareholders in Oxcia AB. The other authors declare no competing interests.
Figures



Similar articles
-
MTH1 Inhibitor TH1579 Induces Oxidative DNA Damage and Mitotic Arrest in Acute Myeloid Leukemia.Cancer Res. 2021 Nov 15;81(22):5733-5744. doi: 10.1158/0008-5472.CAN-21-0061. Epub 2021 Sep 30. Cancer Res. 2021. PMID: 34593524 Free PMC article.
-
Validation and development of MTH1 inhibitors for treatment of cancer.Ann Oncol. 2016 Dec;27(12):2275-2283. doi: 10.1093/annonc/mdw429. Epub 2016 Nov 8. Ann Oncol. 2016. PMID: 27827301
-
AXL and CAV-1 play a role for MTH1 inhibitor TH1579 sensitivity in cutaneous malignant melanoma.Cell Death Differ. 2020 Jul;27(7):2081-2098. doi: 10.1038/s41418-019-0488-1. Epub 2020 Jan 9. Cell Death Differ. 2020. PMID: 31919461 Free PMC article.
-
TH1579, MTH1 inhibitor, delays tumour growth and inhibits metastases development in osteosarcoma model.EBioMedicine. 2020 Mar;53:102704. doi: 10.1016/j.ebiom.2020.102704. Epub 2020 Mar 7. EBioMedicine. 2020. PMID: 32151797 Free PMC article.
-
Potent and specific MTH1 inhibitors targeting gastric cancer.Cell Death Dis. 2019 Jun 4;10(6):434. doi: 10.1038/s41419-019-1665-3. Cell Death Dis. 2019. PMID: 31164636 Free PMC article.
Cited by
-
Pharmacological inhibition of MutT homolog 1 (MTH1) in allergic airway inflammation as a novel treatment strategy.Respir Res. 2025 Mar 14;26(1):101. doi: 10.1186/s12931-025-03175-z. Respir Res. 2025. PMID: 40087604 Free PMC article.
-
Mitotic MTH1 Inhibitors in Treatment of Cancer.Cancer Treat Res. 2023;186:223-237. doi: 10.1007/978-3-031-30065-3_13. Cancer Treat Res. 2023. PMID: 37978139
-
Clinical Significance of NUDT1 (MTH1) Across Cancer Types.Int J Mol Sci. 2025 May 27;26(11):5137. doi: 10.3390/ijms26115137. Int J Mol Sci. 2025. PMID: 40507948 Free PMC article. Review.
-
TH588 and Low-Dose Nocodazole Impair Chromosome Congression by Suppressing Microtubule Turnover within the Mitotic Spindle.Cancers (Basel). 2021 Nov 29;13(23):5995. doi: 10.3390/cancers13235995. Cancers (Basel). 2021. PMID: 34885104 Free PMC article.
-
Targeting the DNA damage response and repair in cancer through nucleotide metabolism.Mol Oncol. 2022 Nov;16(21):3792-3810. doi: 10.1002/1878-0261.13227. Epub 2022 May 28. Mol Oncol. 2022. PMID: 35583750 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

