Novel magnetically retrievable In2O3/MoS2/Fe3O4 nanocomposite materials for enhanced photocatalytic performance
- PMID: 33737582
- PMCID: PMC7973746
- DOI: 10.1038/s41598-021-85532-8
Novel magnetically retrievable In2O3/MoS2/Fe3O4 nanocomposite materials for enhanced photocatalytic performance
Abstract
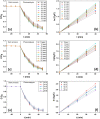
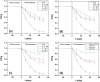
The current work involves synthesis of hybrid nanomaterial of In2O3/MoS2/Fe3O4 and their applications as photocatalysts for disintegration of esomeprazole under visible light illumination. The data emerged from various analyses testified to the successful construction of the desired nano-scaled hybrid photocatalyst. Tauc plot gave the band gap of In2O3/MoS2/Fe3O4 to be ~ 2.15 eV. Synergistic effects of the integrant components enabled efficacious photocatalytic performances of the nanocomposite. The nanohybrid photocatalyst In2O3/MoS2/Fe3O4 showed photodecomposition up to ~ 92.92% within 50 min. The current work realizes its objective of constructing metal oxide based hybrid nano-photocatalyst supported on MoS2 sheets for activity in the visible spectrum, which displayed remarkable capacity of disintegrating emerging persistent organic contaminants and are magnetically recoverable.
Conflict of interest statement
The authors declare no competing interests.
Figures














References
-
- Bahnemann DW, Hilgendorff M, Memming R. Charge carrier dynamics at TiO2 particles: reactivity of free and trapped holes. J. Phys. Chem. B. 1997;101(21):4265–4275. doi: 10.1021/jp9639915. - DOI
-
- Fujishima A, Zhang X, Tryk DA. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008;63(12):515–582. doi: 10.1016/j.surfrep.2008.10.001. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

