Performance of preclinical models in predicting drug-induced liver injury in humans: a systematic review
- PMID: 33737635
- PMCID: PMC7973584
- DOI: 10.1038/s41598-021-85708-2
Performance of preclinical models in predicting drug-induced liver injury in humans: a systematic review
Abstract
Drug-induced liver injury (DILI) causes one in three market withdrawals due to adverse drug reactions, causing preventable human suffering and massive financial loss. We applied evidence-based methods to investigate the role of preclinical studies in predicting human DILI using two anti-diabetic drugs from the same class, but with different toxicological profiles: troglitazone (withdrawn from US market due to DILI) and rosiglitazone (remains on US market). Evidence Stream 1: A systematic literature review of in vivo studies on rosiglitazone or troglitazone was conducted (PROSPERO registration CRD42018112353). Evidence Stream 2: in vitro data on troglitazone and rosiglitazone were retrieved from the US EPA ToxCast database. Evidence Stream 3: troglitazone- and rosiglitazone-related DILI cases were retrieved from WHO Vigibase. All three evidence stream analyses were conducted according to evidence-based methodologies and performed according to pre-registered protocols. Evidence Stream 1: 9288 references were identified, with 42 studies included in analysis. No reported biomarker for either drug indicated a strong hazard signal in either preclinical animal or human studies. All included studies had substantial limitations, resulting in "low" or "very low" certainty in findings. Evidence Stream 2: Troglitazone was active in twice as many in vitro assays (129) as rosiglitazone (60), indicating a strong signal for more off-target effects. Evidence Stream 3: We observed a fivefold difference in both all adverse events and liver-related adverse events reported, and an eightfold difference in fatalities for troglitazone, compared to rosiglitazone. In summary, published animal and human trials failed to predict troglitazone's potential to cause severe liver injury in a wider patient population, while in vitro data showed marked differences in the two drugs' off-target activities, offering a new paradigm for reducing drug attrition in late development and in the market. This investigation concludes that death and disability due to adverse drug reactions may be prevented if mechanistic information is deployed at early stages of drug development by pharmaceutical companies and is considered by regulators as a part of regulatory submissions.
Conflict of interest statement
The authors declare no competing interests.
Figures
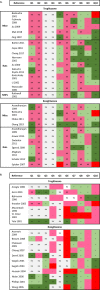



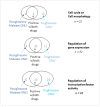

References
-
- US Food and Drug Administration, “Preventable Adverse Drug Reactions: A Focus on Drug Interactions. ADRs: Prevalence and incidence,” https://www.fda.gov/drugs/drug-interactions-labeling/preventable-adverse... (Accessed 24 Mar 2020).
-
- Elliott, R., Camacho, E., Campbell, F., Jankovic, D., St James, M. M., Kaltenthaler, E., & Faria, R. Prevalence and economic burden of medication errors in the NHS in England: rapid evidence synthesis and economic analysis of the prevalence and burden of medication error in the UK www.eepru.org.uk/wp-content/uploads/2020/03/medication-error-report-edit... (Accessed 27 Mar 2020), (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

