Spinal astroglial cannabinoid receptors control pathological tremor
- PMID: 33737752
- PMCID: PMC7610740
- DOI: 10.1038/s41593-021-00818-4
Spinal astroglial cannabinoid receptors control pathological tremor
Abstract
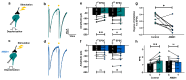
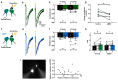
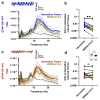
Cannabinoids reduce tremor associated with motor disorders induced by injuries and neurodegenerative disease. Here we show that this effect is mediated by cannabinoid receptors on astrocytes in the ventral horn of the spinal cord, where alternating limb movements are initiated. We first demonstrate that tremor is reduced in a mouse model of essential tremor after intrathecal injection of the cannabinoid analog WIN55,212-2. We investigate the underlying mechanism using electrophysiological recordings in spinal cord slices and show that endocannabinoids released from depolarized interneurons activate astrocytic cannabinoid receptors, causing an increase in intracellular Ca2+, subsequent release of purines and inhibition of excitatory neurotransmission. Finally, we show that the anti-tremor action of WIN55,212-2 in the spinal cords of mice is suppressed after knocking out CB1 receptors in astrocytes. Our data suggest that cannabinoids reduce tremor via their action on spinal astrocytes.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Methods only references
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

