Peripherally Induced Reconditioning of the Central Nervous System: A Proposed Mechanistic Theory for Sustained Relief of Chronic Pain with Percutaneous Peripheral Nerve Stimulation
- PMID: 33737830
- PMCID: PMC7966353
- DOI: 10.2147/JPR.S297091
Peripherally Induced Reconditioning of the Central Nervous System: A Proposed Mechanistic Theory for Sustained Relief of Chronic Pain with Percutaneous Peripheral Nerve Stimulation
Abstract
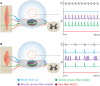
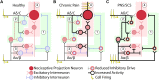
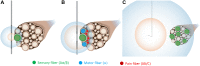
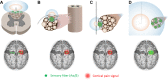
Peripheral nerve stimulation (PNS) is an effective tool for the treatment of chronic pain, although its efficacy and utilization have previously been significantly limited by technology. In recent years, purpose-built percutaneous PNS devices have been developed to overcome the limitations of conventional permanently implanted neurostimulation devices. Recent clinical evidence suggests clinically significant and sustained reductions in pain can persist well beyond the PNS treatment period, outcomes that have not previously been observed with conventional permanently implanted neurostimulation devices. This narrative review summarizes mechanistic processes that contribute to chronic pain, and the potential mechanisms by which selective large diameter afferent fiber activation may reverse these changes to induce a prolonged reduction in pain. The interplay of these mechanisms, supported by data in chronic pain states that have been effectively treated with percutaneous PNS, will also be discussed in support of a new theory of pain management in neuromodulation: Peripherally Induced Reconditioning of the Central Nervous System (CNS).
Keywords: chronic pain; cortical plasticity; mechanism of action; neuromodulation; peripheral nerve stimulation; peripherally induced reconditioning.
© 2021 Deer et al.
Conflict of interest statement
TRD has consulted for Abbott, Axonics, Cornerloc, Ethos, Flowonix, Nalu, Saluda, SpineThera, Stimgenics, SI Bone, Medtronic, PainTeq, Vertiflex (Boston Scientific), Nevro, Vertos, and SPR Therapeutics. He has received research support from Vertiflex, Vertos, Abbott, Mainstay, Saluda, SPR Therapeutics. He holds minor equity in Bioness, Cornerloc, Ethos, Vertiflex, Vertos, Nalu, SpineThera, Saluda, and SPR Therapeutics. SE has consulted for Medtronic, Inc, Mainstay Medical, Boston Scientific Corp, Saluda, and Abbott. He has received research support from the National Institute of Health Research, Medtronic, Inc, and Nevro Corp. SMF has consulted for Abbott, Medtronic, Nevro, Saluda Medical, SPR Therapeutics, and Vertiflex (Boston Scientific). He has received research support from Abbott, Biotronik, Medtronic, Nuvectra, and Saluda Medical. He holds stock options in SpineThera, SPR Therapeutics, Stimgenics, Cornerloc, and Thermaquil, and has equity interest in Saluda Medical. MAH has consulted for SPR, Saluda and Mainstay Medical. PSS has consulted for Abbott, Nevro, Medtronic, and SPR Therapeutics. He has received research support from Boston Scientific, Abbott, Vertos, Nevro, Saluda and Grunenthal Halyard. He is in the Clinical Advisory Board for AIS Therapeutics. He has stock or equity interest in SPR Therapeutics, Nalu and electroCore. IRC, NDC, and JWB are employees of SPR Therapeutics. NDC and JWB report systems and methods for sustained relief of chronic pain patent pending. The authors report no other conflicts of interest in this work.
Figures
References
-
- Gildenberg PL. History of electrical neuromodulation for chronic pain. Pain Med. 2006;7:S7–S13. doi: 10.1111/j.1526-4637.2006.00118.x - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

