Monotherapy With Anti-CD70 Antibody Causes Long-Term Mouse Cardiac Allograft Acceptance With Induction of Tolerogenic Dendritic Cells
- PMID: 33737923
- PMCID: PMC7961176
- DOI: 10.3389/fimmu.2020.555996
Monotherapy With Anti-CD70 Antibody Causes Long-Term Mouse Cardiac Allograft Acceptance With Induction of Tolerogenic Dendritic Cells
Abstract
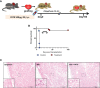
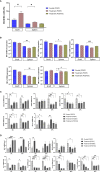
Allograft rejection has been an obstacle for the long-term survival of patients. CD70, a tumor necrosis factor (TNF) family member critically expressed on antigen-presenting cells and strongly but transiently up-regulated during lymphocyte activation, represents an important co-stimulatory molecule that induces effective T cell responses. We used a mouse heterotopic cardiac transplantation model to evaluate the effects of monotherapy with the antibody targeting mouse CD70 (FR70) on transplantation tolerance and its immunoregulatory activity. FR70-treated C3H recipient mice permanently accepted B6 fully mismatched cardiac allografts. Consistent with the graft survival, the infiltration of CD8+ T cells in the graft was reduced, dendritic cells were differentiated into a tolerogenic status, and the number of regulatory T cells was elevated both in the graft and the recipient's spleen. In addition, naïve C3H given an adoptive transfer of spleen cells from the primary recipients with FR70 treatment accepted a heart graft from a matching B6 donor but not third-party BALB/c mice. Our findings show that treatment with FR70 induced regulatory cells and inhibited cytotoxic T cell proliferation, which led to long-term acceptance of mouse cardiac allografts. These findings highlight the potential role of anti-CD70 antibodies as a clinically effective treatment for allograft rejection.
Keywords: CD70; allograft; dendritic cell; regulatory T cell; rejection; tolerance.
Copyright © 2021 Zhao, Que, Du, Fujino, Ichimaru, Ueta, Tokuda, Guo, Zabrocki, de Haard, Nonomura and Li.
Conflict of interest statement
PZ was employed by argenx BV. HH holds ownership interest (including patents) in argenx. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Blockade of costimulatory CD27/CD70 pathway promotes corneal allograft survival.Exp Eye Res. 2020 Oct;199:108190. doi: 10.1016/j.exer.2020.108190. Epub 2020 Aug 14. Exp Eye Res. 2020. PMID: 32798537
-
Simultaneous blockade of co-stimulatory signals, CD28 and ICOS, induced a stable tolerance in rat heart transplantation.Transpl Immunol. 2003 Oct-Nov;12(1):41-8. doi: 10.1016/S0966-3274(03)00016-9. Transpl Immunol. 2003. PMID: 14551031
-
CD70 signaling is critical for CD28-independent CD8+ T cell-mediated alloimmune responses in vivo.J Immunol. 2005 Feb 1;174(3):1357-64. doi: 10.4049/jimmunol.174.3.1357. J Immunol. 2005. PMID: 15661893
-
Manipulation of Regulatory Dendritic Cells for Induction Transplantation Tolerance.Front Immunol. 2020 Oct 14;11:582658. doi: 10.3389/fimmu.2020.582658. eCollection 2020. Front Immunol. 2020. PMID: 33162996 Free PMC article. Review.
-
Immunotherapy with myeloid cells for tolerance induction.Curr Opin Organ Transplant. 2010 Aug;15(4):416-21. doi: 10.1097/MOT.0b013e32833bcf5e. Curr Opin Organ Transplant. 2010. PMID: 20616727 Free PMC article. Review.
Cited by
-
Multi-targeted immunotherapeutics to treat B cell malignancies.J Control Release. 2023 Jun;358:232-258. doi: 10.1016/j.jconrel.2023.04.048. Epub 2023 May 5. J Control Release. 2023. PMID: 37121515 Free PMC article. Review.
-
The CXCL Family Contributes to Immunosuppressive Microenvironment in Gliomas and Assists in Gliomas Chemotherapy.Front Immunol. 2021 Sep 13;12:731751. doi: 10.3389/fimmu.2021.731751. eCollection 2021. Front Immunol. 2021. PMID: 34603309 Free PMC article.
-
Emerging strategies for treating autoimmune disease with genetically modified dendritic cells.Cell Commun Signal. 2024 May 7;22(1):262. doi: 10.1186/s12964-024-01641-7. Cell Commun Signal. 2024. PMID: 38715122 Free PMC article. Review.
-
Coral calcium carried hydrogen ameliorates the severity of non-alcoholic steatohepatitis induced by a choline deficient high carbohydrate fat-free diet in elderly rats.Sci Rep. 2023 Jul 19;13(1):11646. doi: 10.1038/s41598-023-38856-6. Sci Rep. 2023. PMID: 37468618 Free PMC article.
-
CD70 identifies alloreactive T cells and represents a potential target for prevention and treatment of acute GVHD.Blood Adv. 2024 Sep 24;8(18):4900-4912. doi: 10.1182/bloodadvances.2024012909. Blood Adv. 2024. PMID: 39028952 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

