Translational Research in Vitiligo
- PMID: 33737930
- PMCID: PMC7962476
- DOI: 10.3389/fimmu.2021.624517
Translational Research in Vitiligo
Abstract
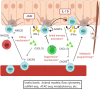
Vitiligo is a disease of the skin characterized by the appearance of white spots. Significant progress has been made in understanding vitiligo pathogenesis over the past 30 years, but only through perseverance, collaboration, and open-minded discussion. Early hypotheses considered roles for innervation, microvascular anomalies, oxidative stress, defects in melanocyte adhesion, autoimmunity, somatic mosaicism, and genetics. Because theories about pathogenesis drive experimental design, focus, and even therapeutic approach, it is important to consider their impact on our current understanding about vitiligo. Animal models allow researchers to perform mechanistic studies, and the development of improved patient sample collection methods provides a platform for translational studies in vitiligo that can also be applied to understand other autoimmune diseases that are more difficult to study in human samples. Here we discuss the history of vitiligo translational research, recent advances, and their implications for new treatment approaches.
Keywords: autoimmunity; genetics; melanocyte oxidative stress; translational research; vitiligo.
Copyright © 2021 Katz and Harris.
Conflict of interest statement
JEH is a consultant for Pfizer, Genzyme/Sanofi, Aclaris Therapeutics Inc, Incyte, Rheos Medicines, Sun Pharmaceuticals, LEO Pharma, Villaris Therapeutics Inc, Dermavant, Temprian, AbbVie Inc, Janssen, TeVido BioDevices, EMD Serono, Almirall, Boston Pharma, Sonoma Biotherapeutics Inc, Methuselah Health, Twi Biotech, Pandion, Cogen Therapeutics Inc, Admirx, BridgeBio, AnaptysBio, Avita, and Frazier Management. JEH is an investigator for Pfizer, Genzyme/Sanofi, Aclaris Therapeutics Inc, Incyte, Rheos Medicines, Sun Pharmaceuticals, LEO Pharma, Villaris Therapeutics Inc, Dermavant, AbbVie, TeVido BioDevices, EMD Serono, and Pandion. JEH is scientific founder of Villaris Therapeutics, Inc, which develops therapeutic treatments for vitiligo, and NIRA Biosciences. JEH is an inventor on patent #62489191 “Diagnosis and Treatment of Vitiligo” that includes targeting IL-15 and Trm for treatment of vitiligo, as well as #067988 “Anti-Human CXCR3 Antibodies for Treatment of Vitiligo” and #029531 “Compositions and Methods for Treating Vitiligo”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

