Safety and efficacy of daclizumab beta in patients with relapsing multiple sclerosis in a 5-year open-label study (EXTEND): final results following early termination
- PMID: 33737954
- PMCID: PMC7934044
- DOI: 10.1177/1756286420987941
Safety and efficacy of daclizumab beta in patients with relapsing multiple sclerosis in a 5-year open-label study (EXTEND): final results following early termination
Abstract
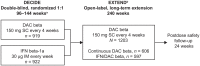
Background: EXTEND (NCT01797965), an open-label extension study, evaluated the safety and efficacy of daclizumab beta in participants with relapsing multiple sclerosis (MS) who had completed the randomized DECIDE study.
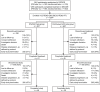
Methods: Eligible participants who received either daclizumab beta or interferon beta-1a in DECIDE received daclizumab beta 150 mg subcutaneously every 4 weeks for up to 5 years in EXTEND, followed by 24 weeks of post-dosing follow-up. Safety and tolerability were evaluated, as were clinical efficacy and magnetic resonance imaging (MRI). EXTEND was terminated ahead of schedule by the sponsors.
Results: The total safety population (N = 1203) received at least one dose of daclizumab beta in EXTEND. In the DECIDE and EXTEND combined periods, the median number of doses of daclizumab beta was 53; median time on treatment was 196 weeks. By 24 September 2018, the end of the study, 110/1203 (9%) participants had completed the protocol-specified treatment period and 1101/1203 (92%) had experienced an adverse event (AE). The most commonly reported AEs were MS relapse, nasopharyngitis, and upper respiratory tract infection. Hepatic events (18%), cutaneous events (45%), and infections (62%) were common treatment-related AEs. The incidence of serious AEs was 29%, most commonly MS relapse and infections. The incidence of immune-mediated disorders was 2%; three of seven were encephalitis. Two of six deaths were considered treatment related. In participants who received continuous daclizumab beta throughout DECIDE and EXTEND, the treatment effects on clinical and MRI outcomes were maintained for up to 6 years.
Conclusion: Results from the combined DECIDE-EXTEND study elucidate outcomes of longer-term treatment with daclizumab beta in the clinical trial setting and underscore the importance of pharmacovigilance with immunomodulatory therapies in the real-world setting.
Keywords: autoimmunity; daclizumab; encephalitis; multiple sclerosis.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest: LK reports funding to his institution (University Hospital Basel) in the last 3 years used exclusively for research support; steering committee, advisory board, and consultancy fees from Actelion, Addex, Bayer, Biogen, Celgene, Genzyme, Merck, Mitsubishi, Novartis, Pfizer, Sanofi, and Santhera; speaker fees from Biogen, Merck, Novartis, Sanofi, and Teva; support of educational activities from Bayer, Biogen, CSL Behring, Genzyme, Merck, Novartis, Sanofi, and Teva; license fees for Neurostatus products; and grants from Bayer, Biogen, European Union, Innosuisse, Merck, Novartis, Roche Research Foundation, Swiss MS Society, and Swiss National Research Foundation. SC reports speaking honoraria from and/or advisory boards for Biogen, Bristol Myers Squibb, EMD Serono, Novartis, Pear Therapeutics, Roche Genentech, and Sanofi Genzyme; and research support from AbbVie, Acorda, Actelion, Biogen, MedDay, Novartis, Roche Genentech, and Sanofi Genzyme. DLA reports consultant fees and/or grants from Acorda, Adelphi, Alkermes, Biogen, Canadian Institutes of Health Research, Celgene, Frequency Therapeutics, Genentech, Genzyme, Immune Tolerance Network, Immunotec, International Progressive MS Alliance, MedDay, Merck Serono, MS Society of Canada, Novartis, Pfizer, Receptos, Roche, and Sanofi and an equity interest in NeuroRx Research. RRR is an employee of and holds stock/stock options in AbbVie. JH was a former employee of and holds stock/stock options in AbbVie. BP was a former employee of and holds stock/stock options in Biogen. SF, SX and WC-B are employees of and hold stock/stock options in Biogen. The EXTEND study and these analyses were funded by Biogen (Cambridge, MA, USA) and AbbVie (Redwood City, CA, USA).
Figures

References
-
- Gold R, Giovannoni G, Selmaj K, et al.. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 2013; 381: 2167–2175. - PubMed
-
- Kappos L, Wiendl H, Selmaj K, et al.. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2015; 373: 1418–1428. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

