Observational cohort study of neurological involvement among patients with SARS-CoV-2 infection
- PMID: 33737955
- PMCID: PMC7934032
- DOI: 10.1177/1756286421993701
Observational cohort study of neurological involvement among patients with SARS-CoV-2 infection
Abstract
Background: A growing number of reports suggest that infection with SARS-CoV-2 often leads to neurological involvement; however, data on the incidence and severity are limited to mainly case reports and retrospective studies.
Methods: This prospective, cross-sectional study of 102 SARS-CoV-2 PCR positive patients investigated the frequency, type, severity and risk factors as well as underlying pathophysiological mechanisms of neurological involvement (NIV) in COVID-19 patients.
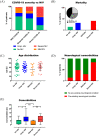
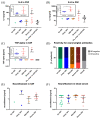
Results: Across the cohort, 59.8% of patients had NIV. Unspecific NIV was suffered by 24.5%, mainly general weakness and cognitive decline or delirium. Mild NIV was found in 9.8%; most commonly, impaired taste or smell. Severe NIV was present in 23.5%; half of these suffered cerebral ischaemia. Incidence of NIV increased with respiratory symptoms of COVID-19. Mortality was higher with increasing NIV severity. Notably, 83.3% with severe NIV had a pre-existing neurological co-morbidity. All cerebrospinal fluid (CSF) samples were negative for SARS-CoV-2 RNA, and SARS-CoV-2 antibody quotient did not suggest intrathecal antibody synthesis. Of the patients with severe NIV, 50% had blood-brain barrier (BBB) disruption and showed a trend of elevated interleukin levels in CSF. Antibodies against neuronal and glial epitopes were detected in 35% of the patients tested.
Conclusion: Cerebrovascular events were the most frequent severe NIV and severe NIV was associated with high mortality. Incidence of NIV increased with respiratory symptoms and NIV and pre-existing neurological morbidities were independent risk factors for fatality. Inflammatory involvement due to BBB disruption and cytokine release drives NIV, rather than direct viral invasion. These findings might help physicians define a further patient group requiring particular attention during the pandemic.
Keywords: COVID-19; Cerebrovascular events; Neuro-COVID.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest: M. Fleischer reports no disclosures relevant to the manuscript. M. Köhrmann reports no disclosures relevant to the manuscript. S. Dolff reports no disclosures relevant to the manuscript. F. Szepanowski reports no disclosures relevant to the manuscript. K. Schmidt reports no disclosures relevant to the manuscript. F. Herbstreit has received speaker honoraria from Biotest and Maquet Getinge. C. Güngör reports no disclosures relevant to the manuscript. B. Stolte reports no disclosures relevant to the manuscript. Ch. Stadtler reports no disclosures relevant to the manuscript. J. Riße reports no disclosures relevant to the manuscript. O. Witzke has received research grants for clinical studies, speaker’s fees, honoraria and travel expenses from Amgen, Alexion, Astellas, Basilea, Biotest, Bristol-Myers Squibb, Correvio, Chiesi, Gilead, Hexal, Janssen, Dr. F. Köhler Chemie, MSD, Novartis, Roche, Pfizer, Sanofi, TEVA and UCB. M. Fiedler reports no disclosures relevant to the manuscript. A. Mausberg reports no disclosures relevant to the manuscript. C. Kill reports no disclosures relevant to the manuscript. G. Meyer zu Horste has received speaker honoraria and served in advisory boards for LFB Pharma. K. Steiner reports no disclosures relevant to the manuscript. M. Forsting reports no disclosures relevant to the manuscript. U. Sure reports no disclosures relevant to the manuscript. U. Dittmer reports no disclosures relevant to the manuscript. T. Brenner reports no disclosures relevant to the manuscript. C. Kleinschnitz reports no disclosures relevant to the manuscript. M. Stettner served on the scientific advisory and/or received speaker honoraria or travel funding from UCB, Biogen Idec; Grifols, Genzyme, Roche, Merck, Novartis, Octapharma, Sanofi-Aventis, TEVA, and Bayer Healthcare. All authors declare approval of the version that is to be published.
Figures

Similar articles
-
SARS-CoV-2 and autoantibodies in the cerebrospinal fluid of COVID-19 patients: prospective multicentre cohort study.Brain Commun. 2023 Oct 17;5(5):fcad274. doi: 10.1093/braincomms/fcad274. eCollection 2023. Brain Commun. 2023. PMID: 37908236 Free PMC article.
-
Myoclonus status revealing COVID 19 infection.Seizure. 2023 Jan;104:12-14. doi: 10.1016/j.seizure.2022.11.010. Epub 2022 Nov 22. Seizure. 2023. PMID: 36446232 Free PMC article.
-
Neurological symptoms in COVID-19: a cross-sectional monocentric study of hospitalized patients.Neurol Res Pract. 2021 Mar 12;3(1):17. doi: 10.1186/s42466-021-00116-1. Neurol Res Pract. 2021. PMID: 33712089 Free PMC article.
-
Neurological manifestations of SARS-CoV-2: complexity, mechanism and associated disorders.Eur J Med Res. 2023 Aug 30;28(1):307. doi: 10.1186/s40001-023-01293-2. Eur J Med Res. 2023. PMID: 37649125 Free PMC article. Review.
-
Neuro-COVID-19: an insidious virus in action.Neurol Neurochir Pol. 2022;56(1):48-60. doi: 10.5603/PJNNS.a2021.0072. Epub 2021 Oct 13. Neurol Neurochir Pol. 2022. PMID: 34642927 Review.
Cited by
-
Anti-GFAP-antibody positive postinfectious acute cerebellar ataxia and myoclonus after COVID-19: a case report.Ther Adv Neurol Disord. 2021 Dec 8;14:17562864211062824. doi: 10.1177/17562864211062824. eCollection 2021. Ther Adv Neurol Disord. 2021. PMID: 34899988 Free PMC article.
-
Taste loss as a distinct symptom of COVID-19: a systematic review and meta-analysis.Chem Senses. 2023 Jan 1;48:bjad043. doi: 10.1093/chemse/bjad043. Chem Senses. 2023. PMID: 38100383 Free PMC article.
-
Building brain capital.Neuron. 2021 May 5;109(9):1430-1432. doi: 10.1016/j.neuron.2021.04.007. Neuron. 2021. PMID: 33957073 Free PMC article.
-
COVID-19-Related Burden and Risk Perception in Individuals with Chronic Inflammatory Demyelinating Polyneuropathy and Multifocal Motor Neuropathy: A Cross-Sectional Study.Neurol Ther. 2022 Sep;11(3):1135-1146. doi: 10.1007/s40120-022-00359-3. Epub 2022 May 12. Neurol Ther. 2022. PMID: 35553393 Free PMC article.
-
COVID-19 outcomes of 10,881 patients: retrospective study of neurological symptoms and associated manifestations (Philippine CORONA Study).J Neural Transm (Vienna). 2021 Nov;128(11):1687-1703. doi: 10.1007/s00702-021-02400-5. Epub 2021 Aug 27. J Neural Transm (Vienna). 2021. PMID: 34448930 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

