Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery
- PMID: 33737963
- PMCID: PMC7934036
- DOI: 10.1177/1758835921996673
Association between tumor molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery
Abstract
Background: Axillary node status is used in clinical practice to guide the selection of axillary surgery in breast cancer patients. However, to date, the optimal axillary management following neoadjuvant therapy (NAT) for breast cancer remains controversial. Our study aimed to investigate the association of molecular subtype, clinical stage, and ypN status after NAT in breast cancer patients, especially those achieving breast pathological complete remission (pCR).
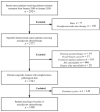
Patients and methods: Patients receiving ⩾4 cycles of NAT were retrospectively included between January 2009 and January 2020. ypN status was compared among patients with different breast pCR statuses, clinical stages, and molecular subtypes in univariate and multivariate analyses.
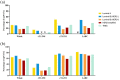
Results: A total of 1999 patients were included: 457 (22.86%), 884 (44.22%), and 658 (32.92%) patients with cT1-2N0, cT1-2N1, and locally advanced breast cancer (LABC), respectively. Altogether, 435 (21.8%) patients achieved breast pCR: 331 with ypN- and 104 with ypN+ status. Patients achieving breast pCR had a significantly lower ypN+ rate than those without pCR [23.9% versus 62.5%, odds ratio (OR) = 0.14, 95% confidence interval (CI) = 0.09-0.21]. For patients with breast pCR, the ypN+ rate was 6.4%, 25.7%, and 33.9% in cT1-2N0, cT1-2N1, and LABC patients, respectively (p < 0.001). Furthermore, the ypN+ rate was 30.8%, 16.8%, 17.5%, 29.6%, and 27.6% in breast pCR patients with the Luminal A, Luminal B (HER2+), HER2-amplified, Luminal B (HER2-), and triple-negative subtype, respectively. Luminal B (HER2+) (OR = 0.20, 95% CI = 0.05-0.82) and HER2-amplified (OR = 0.19, 95% CI = 0.05-0.83) tumors were associated with lower ypN+ rates. Moreover, 100% of breast pCR patients with cT1-2N0 and HER2-positive disease achieved pathological pN0.
Conclusion: In breast pCR patients after NAT, clinical stage and molecular subtype were significantly associated with ypN status. Patients with cT1-2N0 and HER2-positive disease who achieved breast pCR had a very low ypN+ rate, possibly indicating the possibility for de-escalation of axillary surgery in this patient subgroup.
Keywords: breast pathological complete remission; clinical stage; molecular subtype; neoadjuvant therapy; nodal residual burden.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Houssami N, Macaskill P, von Minckwitz G, et al.. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 2012; 48: 3342–3354. - PubMed
-
- von Minckwitz G, Untch M, Blohmer JU, et al.. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012; 30: 1796–1804. - PubMed
-
- Mamounas EP. Impact of neoadjuvant chemotherapy on locoregional surgical treatment of breast cancer. Ann Surg Oncol 2015; 22: 1425–1433. - PubMed
-
- Spring L, Greenup R, Niemierko A, et al.. Pathologic complete response after neoadjuvant chemotherapy and long-term outcomes among young women with breast cancer. J Natl Compr Canc Netw 2017; 15: 1216–1223. - PubMed
-
- Wang-Lopez Q, Chalabi N, Abrial C, et al.. Can pathologic complete response (pCR) be used as a surrogate marker of survival after neoadjuvant therapy for breast cancer? Crit Rev Oncol Hematol 2015; 95: 88–104. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

