Pathophysiology of musculoskeletal pain: a narrative review
- PMID: 33737965
- PMCID: PMC7934019
- DOI: 10.1177/1759720X21995067
Pathophysiology of musculoskeletal pain: a narrative review
Abstract
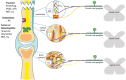
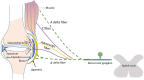
Musculoskeletal pain (excluding bone cancer pain) affects more than 30% of the global population and imposes an enormous burden on patients, families, and caregivers related to functional limitation, emotional distress, effects on mood, loss of independence, and reduced quality of life. The pathogenic mechanisms of musculoskeletal pain relate to the differential sensory innervation of bones, joints, and muscles as opposed to skin and involve a number of peripheral and central nervous system cells and mediators. The interplay of neurons and non-neural cells (e.g. glial, mesenchymal, and immune cells) amplifies and sensitizes pain signals in a manner that leads to cortical remodeling. Moreover, sex, age, mood, and social factors, together with beliefs, thoughts, and pain behaviors influence the way in which musculoskeletal pain manifests and is understood and assessed. The aim of this narrative review is to summarize the different pathogenic mechanisms underlying musculoskeletal pain and how these mechanisms interact to promote the transition from acute to chronic pain.
Keywords: arthralgia; musculoskeletal pain; myalgia; neuroglia; physiology.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: Giustino Varrassi is a member of the journal’s editorial board. The other authors do not have potential conflict of interest to declare.
Figures


References
-
- Treede R, Rief W, Barke A, et al.. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 2019; 160: 19–27. - PubMed
-
- Perrot S, Cohen M, Barke A, et al.. The IASP classification of chronic pain for ICD-11: chronic secondary musculoskeletal pain. Pain 2019; 160: 77–82. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

