Measuring Surrogacy in Clinical Research: With an application to studying surrogate markers for HIV Treatment-as-Prevention
- PMID: 33737982
- PMCID: PMC7962622
- DOI: 10.1007/s12561-019-09244-4
Measuring Surrogacy in Clinical Research: With an application to studying surrogate markers for HIV Treatment-as-Prevention
Abstract
In clinical research, validated surrogate markers are highly desirable in study design, monitoring, and analysis, as they do not only reduce the required sample size and follow-up duration, but also facilitate scientific discoveries. However, challenges exist to identify a reliable marker. One particular statistical challenge arises on how to measure and rank the surrogacy of potential markers quantitatively. We review the main statistical methods for evaluating surrogate markers. In addition, we suggest a new measure, the so-called "population surrogacy fraction of treatment effect," or simply the p-measure, in the setting of clinical trials. The p-measure carries an appealing population impact interpretation and supplements the existing statistical measures of surrogacy by providing "absolute" information. We apply the new measure along with other prominent measures to the HIV Prevention Trial Network 052 Study, a landmark trial for HIV/AIDS treatment-as-prevention.
Keywords: Population attributable fraction; Proportion of treatment effect explained; Randomized trial; Surrogate marker.
Figures
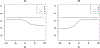
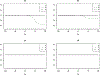

References
-
- Alonso Ariel, Geys Helena, Molenberghs Geert, Michael G Kenward, and Tony Vangeneug-den. Validation of surrogate markers in multiple randomized clinical trials with repeated measurements. Biometrical Journal, 45(8):931–945, 2003. - PubMed
-
- Alonso Ariel, Molenberghs Geert, Burzykowski Tomasz, Renard Didier, Geys Helena, Shkedy Ziv, Tibaldi Fabián, José Cortiñas Abrahantes, and Marc Buyse. Prentice’s Approach and the Meta-Analytic Paradigm: A Reflection on the Role of Statistics in the Evaluation of Surrogate Endpoints. Biometrics, 60(3):724–728, September 2004. - PubMed
-
- Alonso Ariel, Molenberghs Geert, Geys Helena, Buyse Marc, and Vangeneugden Tony. A unifying approach for surrogate marker validation based on prentice’s criteria. Statistics in medicine, 25(2):205–221, 2006. - PubMed
-
- Baker Stuart G. A simple meta-analytic approach for using a binary surrogate endpoint to predict the effect of intervention on true endpoint. Biostatistics, 7(1):58–70, 2005. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
