Evaluating the potential of an amelogenin-derived peptide in tertiary dentin formation
- PMID: 33738118
- PMCID: PMC7955718
- DOI: 10.1093/rb/rbab004
Evaluating the potential of an amelogenin-derived peptide in tertiary dentin formation
Abstract
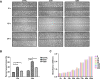
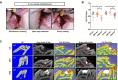
Several novel biomaterials have been developed for dental pulp capping by inducing tertiary dentin formation. The aim of this study was to evaluate the effect of QP5, an amelogenin-based peptide, on the mineralization of dental pulp cells (DPCs) in vitro and in vivo. The cell viability of human DPCs (hDPCs) after treatment with QP5 was determined using the Cell Counting Kit-8 (CCK-8). Migration of hDPCs was assessed using scratch assays, and the pro-mineralization effect was determined using alkaline phosphatase (ALP) staining, alizarin red staining and the expression of mineralization-related genes and proteins. The results showed that QP5 had little effect on the cell viability, and significantly enhanced the migration capability of hDPCs. QP5 promoted the formation of mineralized nodules, and upregulated the activity of ALP, the expression of mRNA and proteins of mineralization-related genes. A pulp capping model in rats was generated to investigate the biological effect of QP5. The results of micro-computed tomography and haematoxylin and eosin staining indicated that the formation of tertiary dentin in QP5-capping groups was more prominent than that in the negative control group. These results indicated the potential of QP5 as a pulp therapy agent.
Keywords: amelogenin-derived peptide; dental pulp cells; pulp capping; tertiary dentin.
© The Author(s) 2021. Published by Oxford University Press.
Figures



Similar articles
-
TVH-19, a synthetic peptide, induces mineralization of dental pulp cells in vitro and formation of tertiary dentin in vivo.Biochem Biophys Res Commun. 2021 Jan 1;534:837-842. doi: 10.1016/j.bbrc.2020.10.095. Epub 2020 Nov 7. Biochem Biophys Res Commun. 2021. PMID: 33168184
-
EDTA Promotes the Mineralization of Dental Pulp In Vitro and In Vivo.J Endod. 2021 Mar;47(3):458-465. doi: 10.1016/j.joen.2020.12.003. Epub 2020 Dec 20. J Endod. 2021. PMID: 33352150
-
Concentrated Growth Factor Promotes Dental Pulp Cells Proliferation and Mineralization and Facilitates Recovery of Dental Pulp Tissue.Med Sci Monit. 2019 Dec 26;25:10016-10028. doi: 10.12659/MSM.919316. Med Sci Monit. 2019. PMID: 31877561 Free PMC article.
-
Odontogenesis by Endocytosis of Peptide Embedding Bioactive Glass Composite.J Dent Res. 2022 Aug;101(9):1055-1063. doi: 10.1177/00220345221085186. Epub 2022 Apr 8. J Dent Res. 2022. PMID: 35394372
-
Potential Roles of Bone Morphogenetic Protein 9 in the Odontogenic Differentiation of Dental Pulp Cells.J Endod. 2021 Mar;47(3):436-443. doi: 10.1016/j.joen.2020.10.018. Epub 2020 Oct 28. J Endod. 2021. PMID: 33129897
Cited by
-
Effect of Recombinant Human Amelogenin on the Osteogenic Differentiation Potential of SHED.Cells. 2025 Apr 30;14(9):657. doi: 10.3390/cells14090657. Cells. 2025. PMID: 40358181 Free PMC article.
-
An amelogenin-based peptide hydrogel promoted the odontogenic differentiation of human dental pulp cells.Regen Biomater. 2022 Jun 17;9:rbac039. doi: 10.1093/rb/rbac039. eCollection 2022. Regen Biomater. 2022. PMID: 35936553 Free PMC article.
-
Physiologic dentin regeneration: its past, present, and future perspectives.Front Physiol. 2023 Dec 11;14:1313927. doi: 10.3389/fphys.2023.1313927. eCollection 2023. Front Physiol. 2023. PMID: 38148896 Free PMC article. Review.
References
-
- Bjorndal L, Simon S, Tomson PL et al. Management of deep caries and the exposed pulp. Int Endod J 2019;52:949–73. - PubMed
-
- Han N, Chen Z, Zhang Q. Expression of KLF5 in odontoblastic differentiation of dental pulp cells during in vitro odontoblastic induction and in vivo dental repair. Int Endod J 2017;50:676–84. - PubMed
-
- Choung HW, Lee DS, Lee J-H et al. Tertiary dentin formation after indirect pulp capping using protein CPNE7. J Dent Res 2016;95:906–12. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

