Characterization and evaluation of a femtosecond laser-induced osseointegration and an anti-inflammatory structure generated on a titanium alloy
- PMID: 33738120
- PMCID: PMC7955712
- DOI: 10.1093/rb/rbab006
Characterization and evaluation of a femtosecond laser-induced osseointegration and an anti-inflammatory structure generated on a titanium alloy
Abstract
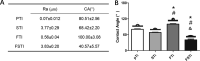
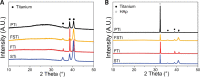
Cell-material interactions during early osseointegration of the bone-implant interface are critical and involve crosstalk between osteoblasts and osteoclasts. The surface properties of titanium implants also play a critical role in cell-material interactions. In this study, femtosecond laser treatment and sandblasting were used to alter the surface morphology, roughness and wettability of a titanium alloy. Osteoblasts and osteoclasts were then cultured on the resulting titanium alloy disks. Four disk groups were tested: a polished titanium alloy (pTi) control; a hydrophilic micro-dislocation titanium alloy (sandblasted Ti (STi)); a hydrophobic nano-mastoid Ti alloy (femtosecond laser-treated Ti (FTi)); and a hydrophilic hierarchical hybrid micro-/nanostructured Ti alloy [femtosecond laser-treated and sandblasted Ti (FSTi)]. The titanium surface treated by the femtosecond laser and sandblasting showed higher biomineralization activity and lower cytotoxicity in simulated body fluid and lactate dehydrogenase assays. Compared to the control surface, the multifunctional titanium surface induced a better cellular response in terms of proliferation, differentiation, mineralization and collagen secretion. Further investigation of macrophage polarization revealed that increased anti-inflammatory factor secretion and decreased proinflammatory factor secretion occurred in the early response of macrophages. Based on the above results, the synergistic effect of the surface properties produced an excellent cellular response at the bone-implant interface, which was mainly reflected by the promotion of early ossteointegration and macrophage polarization.
Keywords: cell–material interactions; femtosecond laser; hybrid micro-/nanostructure; macrophage polarization; osseointegration.
© The Author(s) 2021. Published by Oxford University Press.
Figures










Similar articles
-
Micro/nano topography of selective laser melting titanium inhibits osteoclastogenesis via mediation of macrophage polarization.Biochem Biophys Res Commun. 2021 Dec 3;581:53-59. doi: 10.1016/j.bbrc.2021.09.021. Epub 2021 Sep 15. Biochem Biophys Res Commun. 2021. PMID: 34655976
-
Novel hydrophilic nanostructured microtexture on direct metal laser sintered Ti-6Al-4V surfaces enhances osteoblast response in vitro and osseointegration in a rabbit model.J Biomed Mater Res A. 2016 Aug;104(8):2086-98. doi: 10.1002/jbm.a.35739. Epub 2016 May 4. J Biomed Mater Res A. 2016. PMID: 27086616
-
Manufacture of titanium alloy materials with bioactive sandblasted surfaces and evaluation of osseointegration properties.Front Bioeng Biotechnol. 2023 Aug 21;11:1251947. doi: 10.3389/fbioe.2023.1251947. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37671189 Free PMC article.
-
Nano-scale modification of titanium implant surfaces to enhance osseointegration.Acta Biomater. 2019 Aug;94:112-131. doi: 10.1016/j.actbio.2019.05.045. Epub 2019 May 22. Acta Biomater. 2019. PMID: 31128320 Review.
-
Osseointegration of titanium, titanium alloy and zirconia dental implants: current knowledge and open questions.Periodontol 2000. 2017 Feb;73(1):22-40. doi: 10.1111/prd.12179. Periodontol 2000. 2017. PMID: 28000277 Review.
Cited by
-
Key topographic parameters driving surface adhesion of Porphyromonas gingivalis.Sci Rep. 2023 Sep 23;13(1):15893. doi: 10.1038/s41598-023-42387-5. Sci Rep. 2023. PMID: 37741851 Free PMC article.
-
Micro-/nano-structured zirconia surface promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by reducing pyroptosis under inflammatory conditions.J Dent Sci. 2025 Jul;20(3):1422-1435. doi: 10.1016/j.jds.2025.02.012. Epub 2025 Feb 20. J Dent Sci. 2025. PMID: 40654428 Free PMC article.
-
Influence of Femtosecond Laser Modification on Biomechanical and Biofunctional Behavior of Porous Titanium Substrates.Materials (Basel). 2022 Apr 19;15(9):2969. doi: 10.3390/ma15092969. Materials (Basel). 2022. PMID: 35591307 Free PMC article.
-
Recent advances in regenerative biomaterials.Regen Biomater. 2022 Dec 5;9:rbac098. doi: 10.1093/rb/rbac098. eCollection 2022. Regen Biomater. 2022. PMID: 36518879 Free PMC article. Review.
-
On-Demand Wettability via Combining fs Laser Surface Structuring and Thermal Post-Treatment.Materials (Basel). 2022 Mar 14;15(6):2141. doi: 10.3390/ma15062141. Materials (Basel). 2022. PMID: 35329593 Free PMC article.
References
-
- Tang D, Tare RS, Yang LY et al. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials 2016;83:363–82. - PubMed
-
- Fernandez-Yague MA, Abbah SA, McNamara L et al. Biomimetic approaches in bone tissue engineering: integrating biological and physicomechanical strategies. Adv Drug Deliv Rev 2015;84:1–29. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

