Could Extracellular Vesicles Contribute to Generation or Awakening of "Sleepy" Metastatic Niches?
- PMID: 33738282
- PMCID: PMC7960773
- DOI: 10.3389/fcell.2021.625221
Could Extracellular Vesicles Contribute to Generation or Awakening of "Sleepy" Metastatic Niches?
Abstract
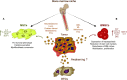
Pre-metastatic niches provide favorable conditions for tumor cells to disseminate, home to and grow in otherwise unfamiliar and distal microenvironments. Tumor-derived extracellular vesicles are now recognized as carriers of key messengers secreted by primary tumors, signals that induce the formation of pre-metastatic niches. Recent evidence suggests that tumor cells can disseminate from the very earliest stages of primary tumor development. However, once they reach distal sites, tumor cells can persist in a dormant state for long periods of time until their growth is reactivated and they produce metastatic lesions. In this new scenario, the question arises as to whether extracellular vesicles could influence the formation of these metastatic niches with dormant tumor cells? (here defined as "sleepy niches"). If so, what are the molecular mechanisms involved? In this perspective-review article, we discuss the possible influence of extracellular vesicles in early metastatic dissemination and whether they might play a role in tumor cell dormancy. In addition, we comment whether extracellular vesicle-mediated signals may be involved in tumor cell awakening, considering the possibility that extracellular vesicles might serve as biomarkers to detect early metastasis and/or minimal residual disease (MRD) monitoring.
Keywords: disseminated tumor cells; dormancy; exosome; extracellular vesicle; metastasis.
Copyright © 2021 Hernández-Barranco, Nogués and Peinado.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alix-Panabières C., Pantel K. (2016). Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 6 479–491. 10.1158/2159-8290.cd-15-1483 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

