Cellular and Supracellular Planar Polarity: A Multiscale Cue to Elongate the Drosophila Egg Chamber
- PMID: 33738289
- PMCID: PMC7961075
- DOI: 10.3389/fcell.2021.645235
Cellular and Supracellular Planar Polarity: A Multiscale Cue to Elongate the Drosophila Egg Chamber
Abstract
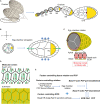
Tissue elongation is known to be controlled by oriented cell division, elongation, migration and rearrangement. While these cellular processes have been extensively studied, new emerging supracellular mechanisms driving tissue extension have recently been unveiled. Tissue rotation and actomyosin contractions have been shown to be key processes driving Drosophila egg chamber elongation. First, egg chamber rotation facilitates the dorsal-ventral alignment of the extracellular matrix and of the cell basal actin fibers. Both fiber-like structures form supracellular networks constraining the egg growth in a polarized fashion thus working as 'molecular corsets'. Second, the supracellular actin fiber network, powered by myosin periodic oscillation, contracts anisotropically driving tissue extension along the egg anterior-posterior axis. During both processes, cellular and supracellular planar polarity provide a critical cue to control Drosophila egg chamber elongation. Here we review how different planar polarized networks are built, maintained and function at both cellular and supracellular levels in the Drosophila ovarian epithelium.
Keywords: actomyosin contractility; planar cell polarity; supracellular network; tissue elongation; tissue rotation.
Copyright © 2021 Popkova, Rauzi and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

