Acetyl-leucine slows disease progression in lysosomal storage disorders
- PMID: 33738443
- PMCID: PMC7954382
- DOI: 10.1093/braincomms/fcaa148
Acetyl-leucine slows disease progression in lysosomal storage disorders
Abstract
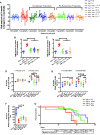
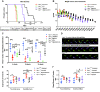
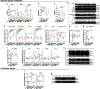
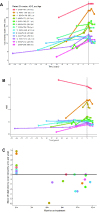
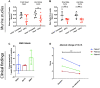
Acetyl-dl-leucine is a derivative of the branched chain amino acid leucine. In observational clinical studies, acetyl-dl-leucine improved symptoms of ataxia, in particular in patients with the lysosomal storage disorder, Niemann-Pick disease type C1. Here, we investigated acetyl-dl-leucine and its enantiomers acetyl-l-leucine and acetyl-d-leucine in symptomatic Npc1-/- mice and observed improvement in ataxia with both individual enantiomers and acetyl-dl-leucine. When acetyl-dl-leucine and acetyl-l-leucine were administered pre-symptomatically to Npc1-/- mice, both treatments delayed disease progression and extended life span, whereas acetyl-d-leucine did not. These data are consistent with acetyl-l-leucine being the neuroprotective enantiomer. Altered glucose and antioxidant metabolism were implicated as one of the potential mechanisms of action of the l-enantiomer in Npc1-/- mice. When the standard of care drug miglustat and acetyl-dl-leucine were used in combination significant synergy resulted. In agreement with these pre-clinical data, when Niemann-Pick disease type C1 patients were evaluated after 12 months of acetyl-dl-leucine treatment, rates of disease progression were slowed, with stabilization or improvement in multiple neurological domains. A beneficial effect of acetyl-dl-leucine on gait was also observed in this study in a mouse model of GM2 gangliosidosis (Sandhoff disease) and in Tay-Sachs and Sandhoff disease patients in individual-cases of off-label-use. Taken together, we have identified an unanticipated neuroprotective effect of acetyl-l-leucine and underlying mechanisms of action in lysosomal storage diseases, supporting its further evaluation in clinical trials in lysosomal disorders.
Keywords: GM2 gangliosidosis; Niemann-Pick disease type C; acetyl-leucine; lysosomal storage diseases; miglustat.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




References
-
- Bremova T, Malinova V, Amraoui Y, Mengel E, Reinke J, Kolnikova M, et al. Acetyl-DL-leucine in Niemann-Pick type C: a case series. Neurology 2015; 85: 1368–75. - PubMed
-
- Bremova-ErtlT, , PlattF, , Strupp M. Sandhoff disease: improvement of gait by acetyl-DL-leucine: A case report. Neuropediatrics 2020; 32892336 - PubMed
-
- Chida J, Yamane K, Takei T, Kido H.. An efficient extraction method for quantitation of adenosine triphosphate in mammalian tissues and cells. Anal Chim Acta 2012; 727: 8–12. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
