Cabazitaxel versus abiraterone or enzalutamide in metastatic castration-resistant prostate cancer: post hoc analysis of the CARD study excluding chemohormonal therapy for castrate-naive disease
- PMID: 33738495
- PMCID: PMC8521736
- DOI: 10.1093/jjco/hyab028
Cabazitaxel versus abiraterone or enzalutamide in metastatic castration-resistant prostate cancer: post hoc analysis of the CARD study excluding chemohormonal therapy for castrate-naive disease
Abstract
Background: In the CARD study (NCT02485691), cabazitaxel significantly improved clinical outcomes versus abiraterone or enzalutamide in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel and the alternative androgen-signalling-targeted inhibitor. However, some patients received docetaxel or the prior alternative androgen-signalling-targeted inhibitor in the metastatic hormone-sensitive (mHSPC) setting. Therefore, the CARD results cannot be directly translated to a Japanese population.
Methods: Patients (N = 255) received cabazitaxel (25 mg/m2 IV Q3W, prednisone, G-CSF) versus abiraterone (1000 mg PO, prednisone) or enzalutamide (160 mg PO) after prior docetaxel and progression ≤12 months on the alternative androgen-signalling-targeted inhibitor. Patients who received combination therapy for mHSPC were excluded (n = 33) as docetaxel is not approved in this setting in Japan.
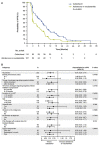
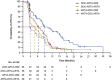
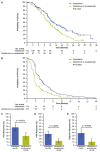
Results: A total of 222 patients (median age 70 years) were included in this subanalysis. Median number of cycles was higher for cabazitaxel versus androgen-signalling-targeted inhibitors (7 versus 4). Clinical outcomes favoured cabazitaxel over abiraterone or enzalutamide including, radiographic progression-free survival (rPFS; median 8.2 versus 3.4 months; P < 0.0001), overall survival (OS; 13.9 versus 11.8 months; P = 0.0102), PFS (4.4 versus 2.7 months; P < 0.0001), confirmed prostate-specific antigen response (37.0 versus 14.4%; P = 0.0006) and objective tumour response (38.9 versus 11.4%; P = 0.0036). For cabazitaxel versus androgen-signalling-targeted inhibitor, grade ≥ 3 adverse events occurred in 55% versus 44% of patients, with adverse events leading to death on study in 2.7% versus 5.7%.
Conclusions: Cabazitaxel significantly improved outcomes including rPFS and OS versus abiraterone or enzalutamide and are reflective of the Japanese patient population. Cabazitaxel should be considered the preferred treatment option over abiraterone or enzalutamide in this setting.
Keywords: chemo-urology; clinical trials; urology.
© The Author(s) 2021. Published by Oxford University Press.
Figures
Similar articles
-
Efficacy and Safety of Cabazitaxel Versus Abiraterone or Enzalutamide in Older Patients with Metastatic Castration-resistant Prostate Cancer in the CARD Study.Eur Urol. 2021 Oct;80(4):497-506. doi: 10.1016/j.eururo.2021.06.021. Epub 2021 Jul 15. Eur Urol. 2021. PMID: 34274136 Clinical Trial.
-
Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): an analysis of a randomised, multicentre, open-label, phase 4 study.Lancet Oncol. 2020 Nov;21(11):1513-1525. doi: 10.1016/S1470-2045(20)30449-6. Epub 2020 Sep 11. Lancet Oncol. 2020. PMID: 32926841 Clinical Trial.
-
Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer.N Engl J Med. 2019 Dec 26;381(26):2506-2518. doi: 10.1056/NEJMoa1911206. Epub 2019 Sep 30. N Engl J Med. 2019. PMID: 31566937 Clinical Trial.
-
Cabazitaxel versus abiraterone or enzalutamide for metastatic castration-resistant prostate cancer following docetaxel failure: a systematic review and meta-analysis.Clin Transl Oncol. 2025 Aug;27(8):3465-3476. doi: 10.1007/s12094-025-03851-y. Epub 2025 Feb 22. Clin Transl Oncol. 2025. PMID: 39987332
-
What do we know about treatment sequencing of abiraterone, enzalutamide, and chemotherapy in metastatic castration-resistant prostate cancer?World J Urol. 2016 May;34(5):617-24. doi: 10.1007/s00345-015-1687-0. Epub 2015 Sep 15. World J Urol. 2016. PMID: 26373956
Cited by
-
The Efficacy of Cabazitaxel in Treating Prostate Cancer: A Systematic Review and Meta-Analysis.Am J Mens Health. 2024 Sep-Oct;18(5):15579883241285162. doi: 10.1177/15579883241285162. Am J Mens Health. 2024. PMID: 39367721 Free PMC article.
-
Lutetium-177 PSMA radioligand therapy in taxan-naive first- and second-line metastatic castration resistant prostate cancer after first-line ARPI therapy.Eur J Nucl Med Mol Imaging. 2025 May;52(6):2015-2022. doi: 10.1007/s00259-025-07076-7. Epub 2025 Jan 13. Eur J Nucl Med Mol Imaging. 2025. PMID: 39804375 Free PMC article.
-
The efficacy and safety of cabazitaxel in the treatment of metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis based on randomized controlled trials.Front Pharmacol. 2025 Jul 17;16:1586650. doi: 10.3389/fphar.2025.1586650. eCollection 2025. Front Pharmacol. 2025. PMID: 40746720 Free PMC article.
References
-
- Parker C, Castro E, Fizazi K, et al. . Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1119–34. - PubMed
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology Prostate Cancer (Version 2.2020). 2020. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed December 2020).
-
- Mottet N, Bergh RCN, Briers E, et al. . EAU - ESTRO - ESUR - SIOG guidelines on prostate cancer 2020. In: European Association of Urology Guidelines, Vol. 2020. Edition. Arnhem, the Netherlands: European Association of Urology Guidelines Office, 2020.
-
- Maines F, Caffo O, Veccia A, Bria E. Sequential use of new agents (NAs) after docetaxel (DOC) first line in metastatic castration-resistant prostate cancer (mCRPC) patients (pts): a pooled-analysis of the published studies. J Clin Oncol 2015;33:Abstract 258.
-
- Angelergues A, Efstathiou E, Gyftaki R, et al. . Results of the FLAC European database of metastatic castration-resistant prostate cancer patients treated with docetaxel, cabazitaxel, and androgen receptor-targeted agents. Clin Genitourin Cancer 2018;16:e777–e84. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

