Disuse Osteoporosis: Clinical and Mechanistic Insights
- PMID: 33738515
- PMCID: PMC9013332
- DOI: 10.1007/s00223-021-00836-1
Disuse Osteoporosis: Clinical and Mechanistic Insights
Abstract
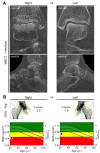
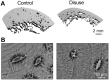
Disuse osteoporosis describes a state of bone loss due to local skeletal unloading or systemic immobilization. This review will discuss advances in the field that have shed light on clinical observations, mechanistic insights and options for the treatment of disuse osteoporosis. Clinical settings of disuse osteoporosis include spinal cord injury, other neurological and neuromuscular disorders, immobilization after fractures and bed rest (real or modeled). Furthermore, spaceflight-induced bone loss represents a well-known adaptive process to microgravity. Clinical studies have outlined that immobilization leads to immediate bone loss in both the trabecular and cortical compartments accompanied by relatively increased bone resorption and decreased bone formation. The fact that the low bone formation state has been linked to high levels of the osteocyte-secreted protein sclerostin is one of the many findings that has brought matrix-embedded, mechanosensitive osteocytes into focus in the search for mechanistic principles. Previous basic research has primarily involved rodent models based on tail suspension, spaceflight and other immobilization methods, which have underlined the importance of osteocytes in the pathogenesis of disuse osteoporosis. Furthermore, molecular-based in vitro and in vivo approaches have revealed that osteocytes sense mechanical loading through mechanosensors that translate extracellular mechanical signals to intracellular biochemical signals and regulate gene expression. Osteocytic mechanosensors include the osteocyte cytoskeleton and dendritic processes within the lacuno-canalicular system (LCS), ion channels (e.g., Piezo1), extracellular matrix, primary cilia, focal adhesions (integrin-based) and hemichannels and gap junctions (connexin-based). Overall, disuse represents one of the major factors contributing to immediate bone loss and osteoporosis, and alterations in osteocytic pathways appear crucial to the bone loss associated with unloading.
Keywords: Bone loss; Immobilization; Microstructure; Osteocyte; Unloading.
© 2021. The Author(s).
Conflict of interest statement
Tim Rolvien and Michael Amling declare that they have no conflict of interest.
Figures


References
-
- Wolff J. Das Gesetz der Transformation der Knochen. Berlin: Hirschwald; 1892.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

