A TRiP Through the Roles of Transient Receptor Potential Cation Channels in Type 2 Upper Airway Inflammation
- PMID: 33738577
- PMCID: PMC7973410
- DOI: 10.1007/s11882-020-00981-x
A TRiP Through the Roles of Transient Receptor Potential Cation Channels in Type 2 Upper Airway Inflammation
Abstract
Purpose of review: Despite their high prevalence, the pathophysiology of allergic rhinitis (AR) and chronic rhinosinusitis (CRS) remains unclear. Recently, transient receptor potential (TRP) cation channels emerged as important players in type 2 upper airway inflammatory disorders. In this review, we aim to discuss known and yet to be explored roles of TRP channels in the pathophysiology of AR and CRS with nasal polyps.
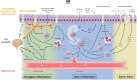
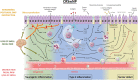
Recent findings: TRP channels participate in a plethora of cellular functions and are expressed on T cells, mast cells, respiratory epithelial cells, and sensory neurons of the upper airways. In chronic upper airway inflammation, TRP vanilloid 1 is mostly studied in relation to nasal hyperreactivity. Several other TRP channels such as TRP vanilloid 4, TRP ankyrin 1, TRP melastatin channels, and TRP canonical channels also have important functions, rendering them potential targets for therapy. The role of TRP channels in type 2 inflammatory upper airway diseases is steadily being uncovered and increasingly recognized. Modulation of TRP channels may offer therapeutic perspectives.
Keywords: Allergic rhinitis; Chronic rhinosinusitis; Mast cell; Nasal hyperreactivity; Respiratory epithelial cell; T cell; Transient receptor potential; Type 2 inflammation.
Conflict of interest statement
The authors declare no conflicts of interest relevant to this manuscript.
Figures
References
-
- Hellings PW, Klimek L, Cingi C, Agache I, Akdis C, Bachert C, Bousquet J, Demoly P, Gevaert P, Hox V, Hupin C, Kalogjera L, Manole F, Mösges R, Mullol J, Muluk NB, Muraro A, Papadopoulos N, Pawankar R, Rondon C, Rundenko M, Seys SF, Toskala E, van Gerven L, Zhang L, Zhang N, Fokkens WJ. Non-allergic rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2017;72(11):1657–1665. - PubMed
-
- •• Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(S29):1–464. Hallmark paper on the pathophysiology and diagnostic and therapeutic approaches towards rhinosinusitis. - PubMed
-
- Sin B, Togias A. Pathophysiology of allergic and nonallergic rhinitis. Proc Am Thorac Soc. 2011;8:106–114. - PubMed
-
- Sarin S, Undem B, Sanico A, Togias A. The role of the nervous system in rhinitis. J Allergy Clin Immunol. 2006;118(5):999–1014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

