Adverse drug reactions in older adults: a narrative review of the literature
- PMID: 33738772
- PMCID: PMC8149349
- DOI: 10.1007/s41999-021-00481-9
Adverse drug reactions in older adults: a narrative review of the literature
Erratum in
-
Correction to: Adverse drug reactions in older adults: a narrative review of the literature.Eur Geriatr Med. 2022 Feb;13(1):307. doi: 10.1007/s41999-021-00591-4. Eur Geriatr Med. 2022. PMID: 34811703 Free PMC article. No abstract available.
Abstract
Purpose: Adverse drug reactions (ADRs) represent a common and potentially preventable cause of unplanned hospitalization, increasing morbidity, mortality, and healthcare costs. We aimed to review the classification and occurrence of ADRs in the older population, discuss the role of age as a risk factor, and identify interventions to prevent ADRs.
Methods: We performed a narrative scoping review of the literature to assess classification, occurrence, factors affecting ADRs, and possible strategies to identify and prevent ADRs.
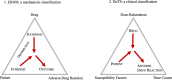
Results: Adverse drug reactions (ADRs) are often classified as Type A and Type B reactions, based on dose and effect of the drugs and fatality of the reaction. More recently, other approaches have been proposed (i.e. Dose, Time and Susceptibility (DoTS) and EIDOS classifications). The frequency of ADRs varies depending on definitions, characteristics of the studied population, and settings. Their occurrence is often ascribed to commonly used drugs, including anticoagulants, antiplatelet agents, digoxin, insulin, and non-steroidal anti-inflammatory drugs. Age-related factors-changes in pharmacokinetics, multimorbidity, polypharmacy, and frailty-have been related to ADRs. Different approaches (i.e. medication review, software identifying potentially inappropriate prescription and drug interactions) have been suggested to prevent ADRs and proven to improve the quality of prescribing. However, consistent evidence on their effectiveness is still lacking. Few studies suggest that a comprehensive geriatric assessment, aimed at identifying individual risk factors, patients' needs, treatment priorities, and strategies for therapy optimization, is key for reducing ADRs.
Conclusions: Adverse drug reactions (ADRs) are a relevant health burden. The medical complexity that characterizes older patients requires a holistic approach to reduce the burden of ADRs in this population.
Keywords: Adverse drug reactions; Frailty; Multimorbidity; Older adults; Polypharmacy.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
References
-
- WHO Meeting on International Drug Monitoring: the Role of National Centres (1971: Geneva, Switzerland) & World Health Organization. (1972). International drug monitoring : the role of national centres , report of a WHO meeting [held in Geneva from 20 to 25 September 1971]. World Health Organization. https://apps.who.int/iris/handle/10665/40968
-
- Management Sciences for Health and World Health Organization . Drug and Therapeutics Committee Training Course. Submitted to the U.S. Agency for International Development by the Rational Pharmaceutical Management Plus Program. Arlington: Management Sciences for Health; 2007.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical