Neutral sphingomyelinase-2 and cardiometabolic diseases
- PMID: 33738905
- PMCID: PMC8365731
- DOI: 10.1111/obr.13248
Neutral sphingomyelinase-2 and cardiometabolic diseases
Abstract
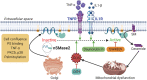
Sphingolipids, in particular ceramides, play vital role in pathophysiological processes linked to metabolic syndrome, with implications in the development of insulin resistance, pancreatic ß-cell dysfunction, type 2 diabetes, atherosclerosis, inflammation, nonalcoholic steatohepatitis, and cancer. Ceramides are produced by the hydrolysis of sphingomyelin, catalyzed by different sphingomyelinases, including neutral sphingomyelinase 2 (nSMase2), whose dysregulation appears to underlie many of the inflammation-related pathologies. In this review, we discuss the current knowledge on the biochemistry of nSMase2 and ceramide production and its regulation by inflammatory cytokines, with particular reference to cardiometabolic diseases. nSMase2 contribution to pathogenic processes appears to involve cyclical feed-forward interaction with proinflammatory cytokines, such as TNF-α and IL-1ß, which activate nSMase2 and the production of ceramides, that in turn triggers the synthesis and release of inflammatory cytokines. We elaborate these pathogenic interactions at the molecular level and discuss the potential therapeutic benefits of inhibiting nSMase2 against inflammation-driven cardiometabolic diseases.
Keywords: cardiometabolic diseases; ceramide; nSMase2; sphingolipid.
© 2021 The Authors. Obesity Reviews published by John Wiley & Sons Ltd on behalf of World Obesity Federation.
Conflict of interest statement
No conflict of interest was declared.
Figures



Similar articles
-
Regulated translocation of neutral sphingomyelinase-2 to the plasma membrane drives insulin resistance in steatotic hepatocytes.J Lipid Res. 2023 Oct;64(10):100435. doi: 10.1016/j.jlr.2023.100435. Epub 2023 Aug 26. J Lipid Res. 2023. PMID: 37640282 Free PMC article.
-
Early activation of nSMase2/ceramide pathway in astrocytes is involved in ischemia-associated neuronal damage via inflammation in rat hippocampi.J Neuroinflammation. 2013 Sep 3;10:109. doi: 10.1186/1742-2094-10-109. J Neuroinflammation. 2013. PMID: 24007266 Free PMC article.
-
Sphingomyelinases and Liver Diseases.Biomolecules. 2020 Oct 30;10(11):1497. doi: 10.3390/biom10111497. Biomolecules. 2020. PMID: 33143193 Free PMC article. Review.
-
Cambinol, a novel inhibitor of neutral sphingomyelinase 2 shows neuroprotective properties.PLoS One. 2015 May 26;10(5):e0124481. doi: 10.1371/journal.pone.0124481. eCollection 2015. PLoS One. 2015. PMID: 26010541 Free PMC article.
-
Roles and regulation of neutral sphingomyelinase-2 in cellular and pathological processes.Adv Biol Regul. 2015 Jan;57:24-41. doi: 10.1016/j.jbior.2014.10.002. Epub 2014 Oct 27. Adv Biol Regul. 2015. PMID: 25465297 Free PMC article. Review.
Cited by
-
Mysterious sphingolipids: metabolic interrelationships at the center of pathophysiology.Front Physiol. 2024 Jan 3;14:1229108. doi: 10.3389/fphys.2023.1229108. eCollection 2023. Front Physiol. 2024. PMID: 38235387 Free PMC article. Review.
-
Involvement of Ceramides in Non-Alcoholic Fatty Liver Disease (NAFLD) Atherosclerosis (ATS) Development: Mechanisms and Therapeutic Targets.Diagnostics (Basel). 2021 Nov 5;11(11):2053. doi: 10.3390/diagnostics11112053. Diagnostics (Basel). 2021. PMID: 34829402 Free PMC article. Review.
-
The Role of Neutral Sphingomyelinase-2 (NSM2) in the Control of Neutral Lipid Storage in T Cells.Int J Mol Sci. 2024 Mar 13;25(6):3247. doi: 10.3390/ijms25063247. Int J Mol Sci. 2024. PMID: 38542220 Free PMC article.
-
Rare genetic variants involved in increased risk of paroxysmal atrial fibrillation in a Japanese population.Sci Rep. 2025 Apr 17;15(1):13216. doi: 10.1038/s41598-025-97794-7. Sci Rep. 2025. PMID: 40240483 Free PMC article.
-
Role of ceramides in diabetic foot ulcers (Review).Int J Mol Med. 2023 Mar;51(3):26. doi: 10.3892/ijmm.2023.5229. Epub 2023 Feb 17. Int J Mol Med. 2023. PMID: 36799149 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

