Enhanced insulin signalling ameliorates C9orf72 hexanucleotide repeat expansion toxicity in Drosophila
- PMID: 33739284
- PMCID: PMC8007214
- DOI: 10.7554/eLife.58565
Enhanced insulin signalling ameliorates C9orf72 hexanucleotide repeat expansion toxicity in Drosophila
Abstract
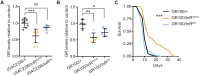
G4C2 repeat expansions within the C9orf72 gene are the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). The repeats undergo repeat-associated non-ATG translation to generate toxic dipeptide repeat proteins. Here, we show that insulin/IGF signalling is reduced in fly models of C9orf72 repeat expansion using RNA sequencing of adult brain. We further demonstrate that activation of insulin/IGF signalling can mitigate multiple neurodegenerative phenotypes in flies expressing either expanded G4C2 repeats or the toxic dipeptide repeat protein poly-GR. Levels of poly-GR are reduced when components of the insulin/IGF signalling pathway are genetically activated in the diseased flies, suggesting a mechanism of rescue. Modulating insulin signalling in mammalian cells also lowers poly-GR levels. Remarkably, systemic injection of insulin improves the survival of flies expressing G4C2 repeats. Overall, our data suggest that modulation of insulin/IGF signalling could be an effective therapeutic approach against C9orf72 ALS/FTD.
Keywords: C9orf72; D. melanogaster; hexanucleotide repeats; insulin signalling; neuroscience.
© 2021, Atilano et al.
Conflict of interest statement
MA, SG, TN, LK, OH, JM, OH, MD, MA, AH, MS, AM, MB, IG, AI, LP No competing interests declared
Figures







References
-
- Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, Craft S, Gandy S, Buettner C, Stoeckel LE, Holtzman DM, Nathan DM. Brain insulin resistance in type 2 diabetes and alzheimer disease: concepts and conundrums. Nature Reviews Neurology. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. - DOI - PMC - PubMed
-
- Augustin H, McGourty K, Allen MJ, Adcott J, Wong CT, Boucrot E, Partridge L. Impact of insulin signaling and proteasomal activity on physiological output of a neuronal circuit in aging Drosophila melanogaster. Neurobiology of Aging. 2018;66:149–157. doi: 10.1016/j.neurobiolaging.2018.02.027. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

