Outcomes and Risk Factors Associated With SARS-CoV-2 Infection in a North American Registry of Patients With Multiple Sclerosis
- PMID: 33739362
- PMCID: PMC7980147
- DOI: 10.1001/jamaneurol.2021.0688
Outcomes and Risk Factors Associated With SARS-CoV-2 Infection in a North American Registry of Patients With Multiple Sclerosis
Erratum in
-
Error in Discussion.JAMA Neurol. 2021 Jun 1;78(6):765. doi: 10.1001/jamaneurol.2021.1236. JAMA Neurol. 2021. PMID: 33938923 Free PMC article. No abstract available.
Abstract
Importance: Emergence of SARS-CoV-2 causing COVID-19 prompted the need to gather information on clinical outcomes and risk factors associated with morbidity and mortality in patients with multiple sclerosis (MS) and concomitant SARS-CoV-2 infections.
Objective: To examine outcomes and risk factors associated with COVID-19 clinical severity in a large, diverse cohort of North American patients with MS.
Design, setting, and participants: This analysis used deidentified, cross-sectional data on patients with MS and SARS-CoV-2 infection reported by health care professionals in North American academic and community practices between April 1, 2020, and December 12, 2020, in the COVID-19 Infections in MS Registry. Health care professionals were asked to report patients after a minimum of 7 days from initial symptom onset and after sufficient time had passed to observe the COVID-19 disease course through resolution of acute illness or death. Data collection began April 1, 2020, and is ongoing.
Exposures: Laboratory-positive SARS-CoV-2 infection or highly suspected COVID-19.
Main outcomes and measures: Clinical outcome with 4 levels of increasing severity: not hospitalized, hospitalization only, admission to the intensive care unit and/or required ventilator support, and death.
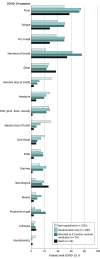
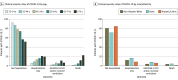
Results: Of 1626 patients, most had laboratory-positive SARS-CoV-2 infection (1345 [82.7%]), were female (1202 [74.0%]), and had relapsing-remitting MS (1255 [80.4%]). A total of 996 patients (61.5%) were non-Hispanic White, 337 (20.8%) were Black, and 190 (11.7%) were Hispanic/Latinx. The mean (SD) age was 47.7 (13.2) years, and 797 (49.5%) had 1 or more comorbidity. The overall mortality rate was 3.3% (95% CI, 2.5%-4.3%). Ambulatory disability and older age were each independently associated with increased odds of all clinical severity levels compared with those not hospitalized after adjusting for other risk factors (nonambulatory: hospitalization only, odds ratio [OR], 2.8 [95% CI, 1.6-4.8]; intensive care unit/required ventilator support, OR, 3.5 [95% CI, 1.6-7.8]; death, OR, 25.4 [95% CI, 9.3-69.1]; age [every 10 years]: hospitalization only, OR, 1.3 [95% CI, 1.1-1.6]; intensive care unit/required ventilator support, OR, 1.3 [95% CI, 0.99-1.7]; death, OR, 1.8 [95% CI, 1.2-2.6]).
Conclusions and relevance: In this registry-based cross-sectional study, increased disability was independently associated with worse clinical severity including death from COVID-19. Other risk factors for worse outcomes included older age, Black race, cardiovascular comorbidities, and recent treatment with corticosteroids. Knowledge of these risk factors may improve the treatment of patients with MS and COVID-19 by helping clinicians identify patients requiring more intense monitoring or COVID-19 treatment.
Conflict of interest statement
Figures
References
-
- Multiple Sclerosis International Federation . Atlas of MS. Published September 2020. Accessed October 24, 2020. http://www.atlasofms.org
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous