Inhibition of Matrix Metalloproteinase 9 Activity Promotes Synaptogenesis in the Hippocampus
- PMID: 33739386
- PMCID: PMC8258443
- DOI: 10.1093/cercor/bhab050
Inhibition of Matrix Metalloproteinase 9 Activity Promotes Synaptogenesis in the Hippocampus
Erratum in
-
Corrigendum to: Inhibition of Matrix Metalloproteinase 9 Activity Promotes Synaptogenesis in the Hippocampus.Cereb Cortex. 2021 May 10;31(6):3161-3163. doi: 10.1093/cercor/bhab105. Cereb Cortex. 2021. PMID: 33834184 Free PMC article. No abstract available.
Abstract
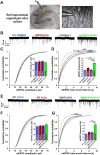
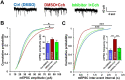
Information coding in the hippocampus relies on the interplay between various neuronal ensembles. We discovered that the application of a cholinergic agonist, carbachol (Cch), which triggers oscillatory activity in the gamma range, induces the activity of matrix metalloproteinase 9 (MMP-9)-an enzyme necessary for the maintenance of synaptic plasticity. Using electrophysiological recordings in hippocampal organotypic slices, we show that Cch potentiates the frequency of miniature inhibitory and excitatory postsynaptic currents (mIPSCs and mEPSCs, respectively) in CA1 neurons and this effect is MMP-9 dependent. Interestingly, though MMP-9 inhibition prevents the potentiation of inhibitory events, it further boosts the frequency of excitatory mEPSCs. Such enhancement of the frequency of excitatory events is a result of increased synaptogenesis onto CA1 neurons. Thus, the function of MMP-9 in cholinergically induced plasticity in the hippocampus is to maintain the fine-tuned balance between the excitatory and the inhibitory synaptic transmission.
Keywords: MMP-9; carbachol; excitatory; inhibitory; synaptogenesis.
© The Author(s) 2021. Published by Oxford University Press.
Figures






References
-
- Behrends JC, ten Bruggencate G. 1993. Cholinergic modulation of synaptic inhibition in the Guinea pig hippocampus in vitro: excitation of GABAergic interneurons and inhibition of GABA-release. J Neurophysiol. 69:626–629. - PubMed
-
- Bland BH, Colom LV. 1993. Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Prog Neurobiol. 41:157–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

