Outcomes on anti-VEGFR-2/paclitaxel treatment after progression on immune checkpoint inhibition in patients with metastatic gastroesophageal adenocarcinoma
- PMID: 33739449
- PMCID: PMC8488901
- DOI: 10.1002/ijc.33559
Outcomes on anti-VEGFR-2/paclitaxel treatment after progression on immune checkpoint inhibition in patients with metastatic gastroesophageal adenocarcinoma
Abstract
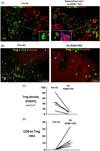
Through our involvement in KEYNOTE-059, we unexpectedly observed durable responses in two patients with metastatic gastroesophageal adenocarcinoma (mGEA) who received ramucirumab (anti-VEGFR-2)/paclitaxel after immune checkpoint inhibition (ICI). To assess the reproducibility of this observation, we piloted an approach to administer ramucirumab/paclitaxel after ICI in more patients, and explored changes in the immune microenvironment. Nineteen consecutive patients with mGEA received ICI followed by ramucirumab/paclitaxel. Most (95%) did not respond to ICI, yet after irRECIST-defined progression on ICI, all patients experienced tumor size reduction on ramucirumab/paclitaxel. The objective response rate (ORR) and progression-free survival (PFS) on ramucirumab/paclitaxel after ICI were higher than on the last chemotherapy before ICI in the same group of patients (ORR, 58.8% vs 11.8%; PFS 12.2 vs 3.0 months; respectively). Paired tumor biopsies examined by imaging mass cytometry showed a median 5.5-fold (range 4-121) lower frequency of immunosuppressive forkhead box P3+ regulatory T cells with relatively preserved CD8+ T cells, post-treatment versus pre-treatment (n = 5 pairs). We then compared the outcomes of these 19 patients with a separate group who received ramucirumab/paclitaxel without preceding ICI (n = 68). Median overall survival on ramucirumab/paclitaxel was longer with (vs without) immediately preceding ICI (14.8 vs 7.4 months) including after multivariate analysis, as was PFS. In our small clinical series, outcomes appeared improved on anti-VEGFR-2/paclitaxel treatment when preceded by ICI, in association with alterations in the immune microenvironment. However, further investigation is needed to determine the generalizability of these data. Prospective clinical trials to evaluate sequential treatment with ICI followed by anti-VEGF(R)/taxane are underway.
Keywords: anti-VEGFR2; gastric/gastroesophageal cancers; immune checkpoint inhibition; paclitaxel; tumor microenvironment.
© 2021 Union for International Cancer Control.
Figures

References
-
- Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. - PubMed
-
- Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;31:1–3. - PubMed
-
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. 10.1016/S1470-2045(14)70420-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

