Favorable Trisomies and ETV6-RUNX1 Predict Cure in Low-Risk B-Cell Acute Lymphoblastic Leukemia: Results From Children's Oncology Group Trial AALL0331
- PMID: 33739852
- PMCID: PMC8274747
- DOI: 10.1200/JCO.20.02370
Favorable Trisomies and ETV6-RUNX1 Predict Cure in Low-Risk B-Cell Acute Lymphoblastic Leukemia: Results From Children's Oncology Group Trial AALL0331
Abstract
Purpose: Children's Oncology Group (COG) AALL0331 tested whether pegaspargase intensification on a low-intensity chemotherapy backbone would improve the continuous complete remission (CCR) rate in a low-risk subset of children with standard-risk B-acute lymphoblastic leukemia (ALL).
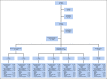
Methods: AALL0331 enrolled 5,377 patients with National Cancer Institute standard-risk B-ALL (age 1-9 years, WBC < 50,000/μL) between 2005 and 2010. Following a common three-drug induction, a cohort of 1,857 eligible patients participated in the low-risk ALL random assignment. Low-risk criteria included no extramedullary disease, < 5% marrow blasts by day 15, end-induction marrow minimal residual disease < 0.1%, and favorable cytogenetics (ETV6-RUNX1 fusion or simultaneous trisomies of chromosomes 4, 10, and 17). Random assignment was to standard COG low-intensity therapy (including two pegaspargase doses, one each during induction and delayed intensification) with or without four additional pegaspargase doses at 3-week intervals during consolidation and interim maintenance. The study was powered to detect a 4% improvement in 6-year CCR rate from 92% to 96%.
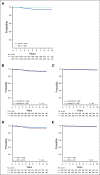
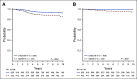
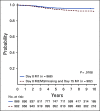
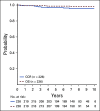
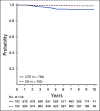
Results: The 6-year CCR and overall survival (OS) rates for the entire low-risk cohort were 94.7% ± 0.6% and 98.7% ± 0.3%, respectively. The CCR rates were similar between arms (intensified pegaspargase 95.3% ± 0.8% v standard 94.0% ± 0.8%; P = .13) with no difference in OS (98.1% ± 0.5% v 99.2% ± 0.3%; P = .99). Compared to a subset of standard-risk study patients given identical therapy who had the same early response characteristics but did not have favorable or unfavorable cytogenetics, outcomes were significantly superior for low-risk patients (CCR hazard ratio 1.95; P = .0004; OS hazard ratio 5.42; P < .0001).
Conclusion: Standard COG therapy without intensified pegaspargase, which can easily be given as an outpatient with limited toxicity, cures nearly all children with B-ALL identified as low-risk by clinical, early response, and favorable cytogenetic criteria.
Conflict of interest statement
Figures
References
-
- Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia J Clin Oncol 1418–241996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

