Quantitative assessment of retinal thickness and vessel density using optical coherence tomography angiography in patients with Alzheimer's disease and glaucoma
- PMID: 33739997
- PMCID: PMC7978346
- DOI: 10.1371/journal.pone.0248284
Quantitative assessment of retinal thickness and vessel density using optical coherence tomography angiography in patients with Alzheimer's disease and glaucoma
Abstract
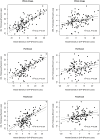
Purpose: Assessment and a direct comparison of retinal vessel density with the thickness of inner retinal layer (IRL) and outer retinal layer (ORL) in the same regions of the macula in subjects with Alzheimer's disease (AD) and primary open-angle glaucoma (POAG).
Methods: We analyzed data from 48 eyes of healthy control (HC) participants, 71 eyes with POAG, and 49 eyes of AD patients. Ophthalmic examination included optical coherence tomography (OCT) imaging to measure IRL and ORL thickness and OCT angiography (OCTA) in the same region for the imaging of vessel density in the superficial vascular plexus (SVP) and deep vascular plexus (DVP) of the retina. A direct comparison of vessel density and retinal layers thickness, which different dynamic ranges, was obtained by normalizing values as percentage losses.
Results: Patients with AD presented significantly greater losses of vascular density in the DVP and ORL thickness compared to POAG (p <0.001), but percentage losses of vessel density in SVP and IRL thickness were considerable in POAG compared to AD eyes (p<0.001). Positive associations among presence of AD were observed primarily in outer retina where a 1% decrease of ORL thickness was associated with about 24-29% increase in odds of the presence of AD. According to OCTA measurements, a 1% decrease of vessel density in DVP was positively associated with a 4-9% increase in odds of the presence of AD. In POAG positive associations among presence of disease were observed only in inner retina where 1% loss of IRL thickness and a 1% loss of vessel density in the SVP were positively associated with a 13-23% increase in risk of presence of the disease.
Conclusions: Analysis of ORL thickness and vessel density in DVP could potentially improve diagnostic capabilities and may provide a valuable approach for predicting of AD.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interest: RK is a paid employee of Sue Ryder Home leading by Pallmed Ltd (https://www.sueryder.org/). There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

