Resident bacteria contribute to opportunistic infections of the respiratory tract
- PMID: 33740012
- PMCID: PMC8011790
- DOI: 10.1371/journal.ppat.1009436
Resident bacteria contribute to opportunistic infections of the respiratory tract
Abstract
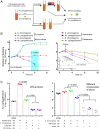
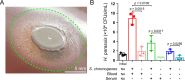
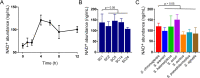
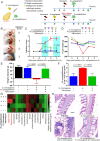
Opportunistic pathogens frequently cause volatile infections in hosts with compromised immune systems or a disrupted normal microbiota. The commensalism of diverse microorganisms contributes to colonization resistance, which prevents the expansion of opportunistic pathogens. Following microbiota disruption, pathogens promptly adapt to altered niches and obtain growth advantages. Nevertheless, whether and how resident bacteria modulate the growth dynamics of invasive pathogens and the eventual outcome of such infections are still unclear. Here, we utilized birds as a model animal and observed a resident bacterium exacerbating the invasion of Avibacterium paragallinarum (previously Haemophilus paragallinarum) in the respiratory tract. We first found that negligibly abundant Staphylococcus chromogenes, rather than Staphylococcus aureus, played a dominant role in Av. paragallinarum-associated infectious coryza in poultry based on epidemic investigations and in vitro analyses. Furthermore, we determined that S. chromogenes not only directly provides the necessary nutrition factor nicotinamide adenine dinucleotide (NAD+) but also accelerates its biosynthesis and release from host cells to promote the survival and growth of Av. paragallinarum. Last, we successfully intervened in Av. paragallinarum-associated infections in animal models using antibiotics that specifically target S. chromogenes. Our findings show that opportunistic pathogens can hijack commensal bacteria to initiate infection and expansion and suggest a new paradigm to ameliorate opportunistic infections by modulating the dynamics of resident bacteria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Virulence of Serovar C-1 Strains of Avibacterium paragallinarum.Avian Dis. 2016 Dec;60(4):837-840. doi: 10.1637/11421-040716-ResNote. Avian Dis. 2016. PMID: 27902901
-
Serovar identification, antimicrobial sensitivity, and virulence of Avibacterium paragallinarum isolated from chickens in Thailand.Avian Dis. 2012 Jun;56(2):359-64. doi: 10.1637/9881-080811-Reg.1. Avian Dis. 2012. PMID: 22856194
-
Coinfection of Avibacterium paragallinarum and Gallibacterium anatis in Specific-Pathogen-Free Chickens Complicates Clinical Signs of Infectious Coryza, Which Can Be Prevented by Vaccination.Avian Dis. 2017 Mar;61(1):55-63. doi: 10.1637/11481-081016-Reg. Avian Dis. 2017. PMID: 28301236
-
Mechanisms of Bacterial Colonization of the Respiratory Tract.Annu Rev Microbiol. 2015;69:425-44. doi: 10.1146/annurev-micro-091014-104209. Annu Rev Microbiol. 2015. PMID: 26488280 Free PMC article. Review.
-
Opportunistic respiratory pathogens in the oral cavity of the elderly.FEMS Immunol Med Microbiol. 2010 Oct;60(1):1-17. doi: 10.1111/j.1574-695X.2010.00709.x. FEMS Immunol Med Microbiol. 2010. PMID: 20579096 Review.
Cited by
-
Antibacterial potential of Propolis: molecular docking, simulation and toxicity analysis.AMB Express. 2024 Jul 16;14(1):81. doi: 10.1186/s13568-024-01741-0. AMB Express. 2024. PMID: 39014110 Free PMC article.
-
Streptococcus pyogenes can support or inhibit growth of Haemophilus influenzae by supplying or restricting extracellular NAD.PLoS One. 2022 Sep 28;17(9):e0270697. doi: 10.1371/journal.pone.0270697. eCollection 2022. PLoS One. 2022. PMID: 36170255 Free PMC article.
-
A detailed analysis of 16S rRNA gene sequencing and conventional PCR-based testing for the diagnosis of bacterial pathogens and discovery of novel bacteria.Antonie Van Leeuwenhoek. 2024 Jul 16;117(1):102. doi: 10.1007/s10482-024-01999-1. Antonie Van Leeuwenhoek. 2024. PMID: 39012584
-
Development of an environmental contamination model to simulate the microbial bloom that occurs in commercial hatch cabinets.Poult Sci. 2022 Jun;101(6):101890. doi: 10.1016/j.psj.2022.101890. Epub 2022 Mar 31. Poult Sci. 2022. PMID: 35512499 Free PMC article.
-
Coumarin Glycosides Reverse Enterococci-Facilitated Enteric Infections.Research (Wash D C). 2024 May 16;7:0374. doi: 10.34133/research.0374. eCollection 2024. Research (Wash D C). 2024. PMID: 38756989 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

