Cell metabolomics analyses revealed a role of altered fatty acid oxidation in neurotoxicity pattern difference between nab-paclitaxel and solvent-based paclitaxel
- PMID: 33740022
- PMCID: PMC7978375
- DOI: 10.1371/journal.pone.0248942
Cell metabolomics analyses revealed a role of altered fatty acid oxidation in neurotoxicity pattern difference between nab-paclitaxel and solvent-based paclitaxel
Abstract
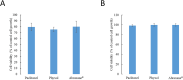
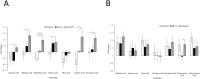
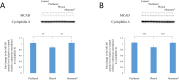
Peripheral neuropathy (PN) is a dose-limiting, painful adverse reaction associated with the use of paclitaxel. This common side effect was often partially attributed to the solvent used for solubilization of the highly hydrophobic drug substance. Therefore, the development of alternative formulations thrived, which included that of Abraxane® containing nanoparticle albumin-bound paclitaxel (nab-paclitaxel). However, studies demonstrated inconsistent conclusions regarding the mitigation of PN in comparison with the traditional formulation. The mass spectrometry-based cell metabolomics approach was used in the present study to explore the potentially associated mechanisms. Although no significant difference in the effects on cell viability was observed, fold changes in carnitine, several acylcarnitines and long-chain fatty acid(s) were significantly different between treatment groups in differentiated and undifferentiated SH-SY5Y cells. The most prominent difference observed was the significant increase of octanoylcarnitine in cells treated with solvent-based paclitaxel, which was found to be associated with significant decrease of medium-chain acyl-CoA dehydrogenase (MCAD). The findings suggested the potential role of altered fatty acid oxidation in the different neurotoxicity patterns observed, which may be a possible target for therapeutic interventions worth further investigation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Jin HW, Flatters SJL, Xiao WH, Mulhern HL, Bennett GJ. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol. 2008;210:229–237. 10.1016/j.expneurol.2007.11.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

