Isolating and Analyzing Protein Containing Granules from Cells
- PMID: 33740275
- PMCID: PMC7988819
- DOI: 10.1002/cpz1.35
Isolating and Analyzing Protein Containing Granules from Cells
Erratum in
-
Group Correction Statement (Data Availability Statements).Curr Protoc. 2022 Aug;2(8):e552. doi: 10.1002/cpz1.552. Curr Protoc. 2022. PMID: 36005902 Free PMC article. No abstract available.
-
Group Correction Statement (Conflict of Interest Statements).Curr Protoc. 2022 Aug;2(8):e551. doi: 10.1002/cpz1.551. Curr Protoc. 2022. PMID: 36005903 Free PMC article. No abstract available.
Abstract
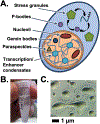
Recent advancements in detection methods have made protein condensates, also called granules, a major area of study, but tools to characterize these assemblies need continued development to keep up with evolving paradigms. We have optimized a protocol to separate condensates from cells using chemical cross-linking followed by size-exclusion chromatography (SEC). After SEC fractionation, the samples can be characterized by a variety of approaches including enzyme-linked immunosorbent assay, dynamic light scattering, electron microscopy, and mass spectrometry. The protocol described here has been optimized for cultured mammalian cells and E. coli expressing recombinant proteins. Since the lysates are fractionated by size, this protocol can be modified to study other large protein assemblies, including the nuclear pore complex, and for other tissues or organisms. © 2021 Wiley Periodicals LLC. Basic Protocol 1: SEC separation of cross-linked mammalian cell lysates Alternate Protocol: Preparation of non-crosslinked mammalian cells Basic Protocol 2: SEC separation of E. coli lysate Support Protocol 1: Detecting protein of interest by ELISA Support Protocol 2: TCA precipitation of SEC fractions.
Keywords: condensates; formaldehyde crosslinking; granule; liquid-liquid phase separation; non-membrane bound organelles; protein assemblies; size-exclusion chromatography.
© 2021 Wiley Periodicals LLC.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

