Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles
- PMID: 33740369
- PMCID: PMC8026680
- DOI: 10.1021/acs.accounts.1c00027
Rhodium-Catalyzed C-H Alkenylation/Electrocyclization Cascade Provides Dihydropyridines That Serve as Versatile Intermediates to Diverse Nitrogen Heterocycles
Abstract
Nitrogen heterocycles are present in approximately 60% of drugs, with nonplanar heterocycles incorporating stereogenic centers being of considerable interest to the fields of medicinal chemistry, chemical biology, and synthetic methods development. Over the past several years, our laboratory has developed synthetic strategies to access highly functionalized nitrogen heterocycles with multiple stereogenic centers. This approach centers on the efficient preparation of diverse 1,2-dihydropyridines by a Rh-catalyzed C-H bond alkenylation/electrocyclization cascade from readily available α,β-unsaturated imines and alkynes. The often densely substituted 1,2-dihydropyridine products have proven to be extremely versatile intermediates that can be elaborated with high regioselectivity and stereoselectivity, often without purification or even isolation. Protonation or alkylation followed by addition of hydride or carbon nucleophiles affords tetrahydropyridines with divergent regioselectivity and stereoselectivity depending on the reaction conditions. Mechanistic experiments in combination with density functional theory (DFT) calculations provide a rationale for the high level of regiocontrol and stereocontrol that is observed. Further elaboration of the tetrahydropyridines by diastereoselective epoxidation and regioselective ring opening furnishes hydroxy-substituted piperidines. Alternatively, piperidines can be obtained directly from dihydropyridines by catalytic hydrogenation in good yields with high face selectivity.When trimethylsilyl alkynes or N-trimethylsilylmethyl imines are employed as starting inputs, the Rh-catalyzed C-H bond alkenylation/electrocyclization cascade provides silyl-substituted dihydropyridines that enable a host of new and useful transformations to different heterocycle classes. Protonation of these products under acidic conditions triggers the loss of the silyl group and the formation of unstabilized azomethine ylides that would be difficult to access by other means. Depending on the location of the silyl group, [3 + 2] cycloaddition of the azomethine ylides with dipolarophiles provides tropane or indolizidine privileged frameworks, which for intramolecular cycloadditions yield complex polycyclic products with up to five contiguous stereogenic centers. When different types of conditions are employed, loss of the silyl group can result in either rearrangement to cyclopropyl-fused pyrrolidines or to aminocyclopentadienes. Mechanistic experiments supported by DFT calculations provide reaction pathways for these unusual rearrangements.The transformations described in this Account are amenable to natural product synthesis and drug discovery applications because of the biological relevance of the structural motifs that are prepared, short reaction sequences that rely on readily available starting inputs, high regiocontrol and stereocontrol, and excellent functional group compatibility. For example, the methods have been applied to efficient asymmetric syntheses of morphinan drugs, including the opioid antagonist (-)-naltrexone, which is extensively used for the treatment of drug abuse.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
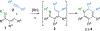
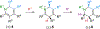
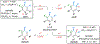
References
-
- Duttwyler S; Chen S; Takase MK; Wiberg KB; Bergman RG; Ellman JA Proton Donor Acidity Controls Selectivity in Nonaromatic Nitrogen Heterocycle Synthesis. Science 2013, 339, 678–682. - PMC - PubMed
-
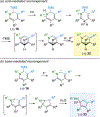
Divergent regioselective and stereoselective elaboration of dihydropyridines give highly functionalized tetrahydropyridines. The dihydropyridines are prepared in a single step by a Rh-catalyzed C−H bond alkenylation/electrocyclization cascade using readily available alkyne and α,β-unsaturated imine inputs.
-
- Chen S; Bacauanu V; Knecht T; Mercado BQ; Bergman RG; Ellman JA New Regio- and Stereoselective Cascades via Unstabilized Azomethine Ylide Cycloadditions for the Synthesis of Highly Substituted Tropane and Indolizidine Frameworks. J. Am. Chem. Soc 2016, 138, 12664–12670. - PMC - PubMed
-
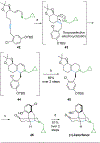
Readily prepared silyl substituted dihydropyridines undergo protonation and alkylation triggered desilylation to give unstabilized azomethine ylides that upon reaction with dipolarophiles provide indolizidines, tropanes, and complex polycyclic heterocycles in good yields and with high regioselectivity and diastereoselectivity.
-
- Walker MM; Koronkiewicz B; Chen S; Houk KN; Mayer JM; Ellman JA Highly Diastereoselective Functionalization of Piperidines by Photoredox-Catalyzed α-Amino C–H Arylation and Epimerization. J. Am. Chem. Soc 2020, 142, 8194–8202. - PMC - PubMed
-
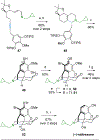
Face-selective catalytic hydrogenation of N-aryl 1,2-dihydropyridines affords piperidines with high stereoselectivity. Photoredox catalyzed C–H arylation of the piperidines with cyanoarenes initially proceeds with poor stereoselectivity, but in situ photoredox-mediated epimerization provides the more stable isomer of the arylated product.
-
- Dongbang S; Pedersen B; Ellman JA Asymmetric Synthesis of (−)-Naltrexone. Chem. Sci 2019, 10, 535–541. - PMC - PubMed
-
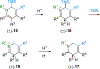
A Rh(I)-catalyzed intramolecular C–H alkenylation and torquoselective electrocyclization cascade affords the hexahydroisoquinoline bicyclic framework that upon elaboration efficiently provides the morphinan core and requisite functionality of (−)-naltrexone, an opioid antagonist used to manage drug abuse.
-
- Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

