Simultaneous binding of Guidance Cues NET1 and RGM blocks extracellular NEO1 signaling
- PMID: 33740419
- PMCID: PMC8063088
- DOI: 10.1016/j.cell.2021.02.045
Simultaneous binding of Guidance Cues NET1 and RGM blocks extracellular NEO1 signaling
Abstract
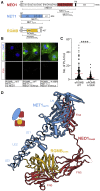
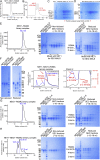
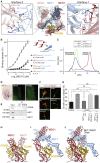
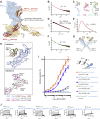
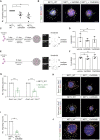
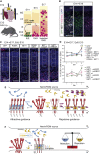
During cell migration or differentiation, cell surface receptors are simultaneously exposed to different ligands. However, it is often unclear how these extracellular signals are integrated. Neogenin (NEO1) acts as an attractive guidance receptor when the Netrin-1 (NET1) ligand binds, but it mediates repulsion via repulsive guidance molecule (RGM) ligands. Here, we show that signal integration occurs through the formation of a ternary NEO1-NET1-RGM complex, which triggers reciprocal silencing of downstream signaling. Our NEO1-NET1-RGM structures reveal a "trimer-of-trimers" super-assembly, which exists in the cell membrane. Super-assembly formation results in inhibition of RGMA-NEO1-mediated growth cone collapse and RGMA- or NET1-NEO1-mediated neuron migration, by preventing formation of signaling-compatible RGM-NEO1 complexes and NET1-induced NEO1 ectodomain clustering. These results illustrate how simultaneous binding of ligands with opposing functions, to a single receptor, does not lead to competition for binding, but to formation of a super-complex that diminishes their functional outputs.
Keywords: Neogenin; Netrin; axon regeneration; cell migration; cell surface receptors; complex structure; morphogen signaling; protein-protein interactions; repulsive guidance molecule; signal transduction.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures










References
-
- Agirre J., Davies G., Wilson K., Cowtan K. Carbohydrate anomalies in the PDB. Nat. Chem. Biol. 2015;11:303. - PubMed
-
- Agirre J., Iglesias-Fernández J., Rovira C., Davies G.J., Wilson K.S., Cowtan K.D. Privateer: software for the conformational validation of carbohydrate structures. Nat. Struct. Mol. Biol. 2015;22:833–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

