Dynamics of adrenergic signaling in cardiac myocytes and implications for pharmacological treatment
- PMID: 33740423
- PMCID: PMC8650805
- DOI: 10.1016/j.jtbi.2021.110619
Dynamics of adrenergic signaling in cardiac myocytes and implications for pharmacological treatment
Abstract
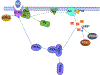
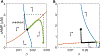
Dense innervation of the heart by the sympathetic nervous system (SNS) allows cardiac output to respond appropriately to the needs of the body under varying conditions, but occasionally the abrupt onset of SNS activity can trigger cardiac arrhythmias. Sympathetic activity leads to the release of norepinephrine (NE) onto cardiomyocytes, activating β1-adrenergic receptors (β1-ARs) and leading to the production of the second messenger cyclic AMP (cAMP). Upon sudden activation of β1-ARs in experiments, intracellular cAMP can transiently rise to a high concentration before converging to a steady state level. Although changes to cellular cAMP concentration are important in modulating the overall cardiovascular response to sympathetic tone, the underlying mechanisms of the cAMP transients and the parameters that control their magnitude are unclear. We reduce a detailed computational model of the β1-adrenergic signaling cascade to a system of two differential equations by eliminating extraneous variables and applying quasi-steady state approximation. The structure of the reduced model reveals that the large cAMP transients associated with abrupt β1-AR activation are generated by the interplay of production/degradation of cAMP and desensitization/resensitization of β1-ARs. The reduced model is used to predict how the dynamics of intracellular cAMP depend on the concentrations of norepinephrine (NE), phosphodiesterases 3 and 4 (PDE3,4), G-protein coupled receptor kinase 2 (GRK2), and β1-AR, in healthy conditions and a simple model of early stages of heart failure. The key findings of the study are as follows: 1) Applying a reduced model of the dynamics of cardiac sympathetic signaling we show that the concentrations of two variables, cAMP and non-desensitized β1-AR, capture the overall dynamics of sympathetic signaling; 2) The key factors influencing cAMP production are AC activity and PDE3,4 activity, while those that directly impact β1-AR phosphorylation are GRK2 and PKA1. Thus, disease states that affect sympathetic control of the heart can be thoroughly assessed by studying AC activity, PDE3,4, GRK2 and PKA activity, as these factors directly impact cAMP production/degradation and β1-AR (de) phosphorylation and are therefore predicted to comprise the most effective pharmaceutical targets in diseases affecting cardiac β1-adrenergic signaling.
Keywords: -blockers; Cyclic AMP; Heart failure; Mathematical model; Sympathetic nervous system.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

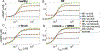


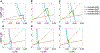
References
-
- Bers DM, 2008. Calcium cycling and signaling in cardiac myocytes. Annual Review of Physiology 70 (1), 23–49. URL:http://www.annualreviews.org/doi/10.1146/annurev.physiol.70.113006.100455. - DOI - PubMed
-
- Bohm M, Maack C, 2000. Treatment of heart failure with beta-blockers. Mechanisms and results. Basic Research in Cardiology 95, 38. - PubMed
-
- Bouzamondo A, Hulot J-S, Sanchez P, Lechat P, 2003. Beta-blocker benefit according to severity of heart failure. European Journal of Heart Failure 5 (3), 281–289. URL: http://doi.wiley.com/10.1016/S1388-9842%2803%2900042-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

