Novel approaches for vaccine development
- PMID: 33740454
- PMCID: PMC8049514
- DOI: 10.1016/j.cell.2021.02.030
Novel approaches for vaccine development
Abstract
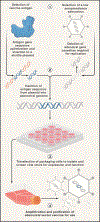
Vaccines are critical tools for maintaining global health. Traditional vaccine technologies have been used across a wide range of bacterial and viral pathogens, yet there are a number of examples where they have not been successful, such as for persistent infections, rapidly evolving pathogens with high sequence variability, complex viral antigens, and emerging pathogens. Novel technologies such as nucleic acid and viral vector vaccines offer the potential to revolutionize vaccine development as they are well-suited to address existing technology limitations. In this review, we discuss the current state of RNA vaccines, recombinant adenovirus vector-based vaccines, and advances from biomaterials and engineering that address these important public health challenges.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.A.B., D.K.E., and A.C. are employees of Moderna Inc. and hold equities from the company. D.H.B. is a co-inventor on SARS-CoV-2 vaccine patents that have been licensed.
Figures




References
-
- Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, et al. (2018). Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med 379, 11–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

