The landscape of autosomal-recessive pathogenic variants in European populations reveals phenotype-specific effects
- PMID: 33740458
- PMCID: PMC8059335
- DOI: 10.1016/j.ajhg.2021.03.004
The landscape of autosomal-recessive pathogenic variants in European populations reveals phenotype-specific effects
Abstract
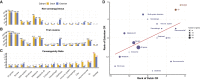
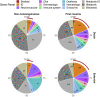
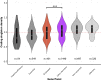
The number and distribution of recessive alleles in the population for various diseases are not known at genome-wide-scale. Based on 6,447 exome sequences of healthy, genetically unrelated Europeans of two distinct ancestries, we estimate that every individual is a carrier of at least 2 pathogenic variants in currently known autosomal-recessive (AR) genes and that 0.8%-1% of European couples are at risk of having a child affected with a severe AR genetic disorder. This risk is 16.5-fold higher for first cousins but is significantly more increased for skeletal disorders and intellectual disabilities due to their distinct genetic architecture.
Keywords: at-risk couples; autosomal recessive disorders; carrier frequency; pre-conception carrier screening; selection.
Copyright © 2021 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
S.C. is a paid consultant to MyHeritage. All other authors declare no competing interests.
Figures


References
-
- Kondrashov A.S. Contamination of the genome by very slightly deleterious mutations: why have we not died 100 times over? J. Theor. Biol. 1995;175:583–594. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

