Adjunctive host-directed therapies for pulmonary tuberculosis: a prospective, open-label, phase 2, randomised controlled trial
- PMID: 33740465
- PMCID: PMC8332197
- DOI: 10.1016/S2213-2600(20)30448-3
Adjunctive host-directed therapies for pulmonary tuberculosis: a prospective, open-label, phase 2, randomised controlled trial
Erratum in
-
Correction to Lancet Respir Med 2021; published online Feb 26. https://doi.org/10.1016/S2213-2600(20)30448-3.Lancet Respir Med. 2021 Jun;9(6):e55. doi: 10.1016/S2213-2600(21)00204-6. Epub 2021 Apr 19. Lancet Respir Med. 2021. PMID: 33887247 Free PMC article. No abstract available.
Abstract
Background: Current tuberculosis treatments leave patients with clinically significant lung injury and increased all-cause mortality post-cure. Adjunctive host-directed therapies could protect the lungs, improve long-term survival, and shorten treatment duration; however, few have been tested clinically. Therefore, we aimed to assess the safety and preliminary efficacy of four host-directed therapies for tuberculosis.
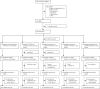
Methods: In this prospective, open-label, phase 2, randomised controlled trial, patients with pulmonary tuberculosis were recruited at three clinical sites in South Africa. Eligible patients were aged 18-65 years, HIV-1-negative, and had rifampicin-susceptible Mycobacterium tuberculosis, a sputum Xpert cycle threshold of less than 20, and moderately advanced or far advanced disease on chest radiography. By use of numbers generated in blocks of ten and stratification by site, eligible patients were randomly assigned (1:1:1:1:1) to receive one of the four oral host-directed treatments plus standard tuberculosis treatment or standard treatment alone (the control group). Host-directed treatments were: CC-11050 (200 mg twice daily, taken with food; day 1-112); everolimus (0·5 mg/day; day 1-112); auranofin (3 mg/day for seven doses, then 6 mg/day; day 1-112); and ergocalciferol (5 mg on day 1, then 2·5 mg on day 28 and day 56). All study participants received oral rifabutin-substituted standard tuberculosis treatment for 180 days. Patients and clinicians were not masked to treatment assignment. Spirometry and sputum culture with solid and liquid media were done at baseline and up to 180 days at specified intervals throughout treatment. The primary endpoint was safety and tolerability up to day 210. Secondary preliminary efficacy endpoints were treatment effects on sputum microbiology (culture status at day 56 and the hazard ratio for stable culture conversion up to day 180) and lung function (FEV1 and forced vital capacity [FVC]) measured by spirometry at day 56, day 180, and day 540. Safety was analysed in the intention-to-treat population and preliminary efficacy primarily in the per-protocol population. The trial is registered at ClinicalTrials.gov, NCT02968927. Post-treatment follow-up was completed in 2020.
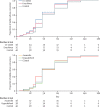
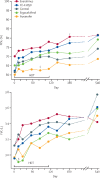
Findings: Between Nov 18, 2016, and Sept 27, 2018, 200 patients were screened and randomly assigned to different treatment groups (n=40 per group, apart from n=39 in the everolimus group after one patient withdrew consent). 11 treatment-emergent serious adverse events occurred either during treatment or within 30 days after treatment discontinuation, of which three were attributable to a host-directed treatment. Life-threatening thrombocytopenia occurred in an auranofin recipient; apparent intra-abdominal sepsis leading to death occurred in another auranofin recipient and was classified as a suspected unexpected serious adverse reaction. Tuberculous spondylitis occurred as an apparent paradoxical reaction in a patient receiving ergocalciferol. Two patients in the control group had life-threatening, treatment-attributable liver injury. No treatment-emergent, treatment-attributable serious adverse events occurred in patients receiving CC-11050 or everolimus. Mean FEV1 in the control group was 61·7% of predicted (95% CI 56·3-67·1) at baseline and 69·1% (62·3-75·8) at day 180. Patients treated with CC-11050 and everolimus had increased recovery of FEV1 at day 180 relative to the control group (mean difference from control group 6·30%, 95% CI 0·06-12·54; p=0·048; and 6·56%, 0·18-12·95; p=0·044, respectively), whereas auranofin and ergocalciferol recipients did not. None of the treatments had an effect on FVC during 180 days of follow-up or on measures of sputum culture status over the course of the study.
Interpretation: CC-11050 and everolimus were safe and reasonably well tolerated as adjunctive therapies for tuberculosis, and analysis of preliminary efficacy suggests they might also enhance the recovery of FEV1, a key measure of lung function and predictor of all-cause mortality. Further studies of these candidates are warranted.
Funding: The Bill & Melinda Gates Foundation and the South African Medical Research Council.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
A leap forward in assessing host-directed therapies for tuberculosis.Lancet Respir Med. 2021 Aug;9(8):809-810. doi: 10.1016/S2213-2600(20)30528-2. Epub 2021 Mar 16. Lancet Respir Med. 2021. PMID: 33740467 Free PMC article. No abstract available.
References
-
- WHO Global tuberculosis report. 2019. https://www.who.int/teams/global-tuberculosis-programme/data
-
- Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med. 1989;83:195–198. - PubMed
-
- Duong M, Islam S, Rangarajan S. Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Health. 2019;7:e613–e623. - PubMed
-
- Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol. 2015;15:255–263. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

