Rethinking prognostic factors in locally advanced or metastatic urothelial carcinoma in the immune checkpoint blockade era: a multicenter retrospective study
- PMID: 33740735
- PMCID: PMC7980066
- DOI: 10.1016/j.esmoop.2021.100090
Rethinking prognostic factors in locally advanced or metastatic urothelial carcinoma in the immune checkpoint blockade era: a multicenter retrospective study
Abstract
Background: Few studies have investigated the safety and efficacy of anti-PD-(L)1 antibodies in metastatic urothelial carcinoma (mUC) in daily clinical practice. Knowledge about the influence of baseline clinical and analytical factors on therapy outcomes is scarce.
Patients and methods: We conducted a multicenter retrospective study involving 119 previously treated or untreated mUC patients under anti-PD-(L)1 therapy in a real-world scenario. The objectives of this study were to confirm the safety and efficacy of anti-PD-(L)1 monotherapy and to identify pretreatment factors influencing therapy outcomes. In addition, an independent prognostic model for overall survival (OS) was developed and internally validated.
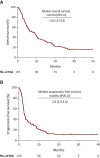
Results: Median OS was 7.8 months [95% confidence interval (CI), 5.4-10.4], median progression-free survival (PFS) was 2.80 months (95% CI, 2.4-3.4), disease control rate (DCR) was 40% (95% CI, 31-49), and overall response rate (ORR) was 24% (95% CI, 15-31). Presence of peritoneal metastases was associated with poor OS [hazard ratio (HR) = 2.40, 95% CI, 1.08-5.33; P = 0.03]. Use of proton-pump inhibitors (PPI) was associated with poor OS (HR = 1.83, 95% CI, 1.11-3.02; P = 0.02) and PFS (HR = 1.94, 95% CI, 1.22-3.09; P = 0.005), and lower DCR (OR = 0.38, 95% CI, 0.17-0.89; P = 0.03) and ORR (OR = 0.18, 95% CI, 0.02-1.60; P = 0.002). The three risk category prognostic model developed included Eastern Cooperative Oncology Group performance status, PPI use, albumin level, presence of liver metastases, and presence of peritoneal metastases variables and was associated with higher risk of death (HR = 3.00, 95% CI, 1.97-4.56; P = 0.0001).
Conclusions: This study confirms anti-PD-(L)1 monotherapy as a safe and effective treatment option in daily clinical practice for mUC patients. It also describes the presence of peritoneal metastases as an independent prognostic factor for OS and underlines the association between PPI use and worse therapeutic outcomes. Finally, it proposes a new easy-to-use risk-assessment model for OS prediction.
Keywords: immunotherapy; peritoneum; prognosis; proton-pump inhibitors; real world; urothelial carcinoma.
Copyright © 2021 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure JR-B: travel, accommodations, expenses: Bristol-Myers Squibb, Merck Sharp & Dohme, Ipsen, PharmaMar, and Roche; honoraria for educational activities: Roche; honoraria for consultancies: Boehringer Ingelheim; institutional research funding: Roche. AM-D: travel, accommodations, expenses: Novartis, Pierre Fabre, Bristol-Myers Squibb, Merck Sharp & Dohme, PharmaMar, Roche, Pfizer, Novartis, Bayer, Ipsen, Eisai, Janssen, Astellas, Sanofi, Lilly, Amgen, Kyowa-Kirin, Boehringer Ingelheim, and AstraZeneca; honoraria for educational activities: Novartis, Pierre Fabre, Bristol-Myers Squibb, Merck Sharp & Dohme, PharmaMar, Roche, Pfizer, Novartis, Bayer, Ipsen, Eisai, Janssen, Astellas, Sanofi, Lilly, Amgen, Kyowa-Kirin, Boehringer Ingelheim, and AstraZeneca; honoraria for consultancies: Pierre Fabre, PharmaMar, Pfizer, Merck Sharp & Dohme; institutional research funding: Merck Sharp & Dohme, and Bristol-Myers Squibb. OF-C: travel, accommodations, expenses: Bristol-Myers Squibb, Ipsen, and Astellas; honoraria for educational activities: Astellas, Roche, Pfizer, Bristol-Myers Squibb, Sanofi, and EUSA Pharma; honoraria educational activities: Pierre Fabre, Novartis, Bristol-Myers Squibb, Roche, Astellas, Bayer, and Janssen. LS: travel, accommodations, expenses: Roche, Pfizer, and Ipsen; honoraria for educational activities: Roche; honoraria for consultancies: Bristol-Myers Squibb and Boehringer Ingelheim. ML-Q: travel, accommodations, expenses: Roche, Merck, Lilly, Pfizer, Ipsen, Boehringer Ingelheim, and Takeda; honoraria for educational activities: Roche, Merck, AstraZeneca, Lilly, Janssen, Ipsen, and Boehringer Ingelheim; honoraria for consultancies: Roche, MSD Oncology, Bristol-Myers Squibb, GSK, Ipsen, Boehringer Ingelheim, Takeda, Sanofi, and Tesaro. RL-L: travel, accommodations, expenses: Lilly, Novartis, Pfizer, Merck, Roche, and Bristol-Myers Squibb; honoraria for educational activities: Lilly, Novartis, Pfizer, Merck, Roche, and Bristol-Myers Squibb; honoraria for consultancies: PharmaMar, Bayer, and Pierre Fabre. SV: travel, accommodations, expenses: Pfizer, Roche, and AstraZeneca; honoraria for consultancies/educational activities: Pfizer, Lilly, Astellas, Janssen, MSD Oncology, Bayer, Roche, Bristol-Myers Squibb, Boehringer Ingelheim, AstraZeneca, Ipsen, Novartis, EUSA Pharma, Eisai, and Sanofi. UA-H: travel, accommodations, expenses: Novartis, Pierre Fabre, Bristol-Myers Squibb, Roche, Pfizer, Bayer, Ipsen, Eisai, Janssen, Astellas, and Sanofi & Kyowa-Kirin; honoraria for educational activities: Sanofi and Ipsen; honoraria for consultancies: Novartis, Pfizer, Bayer, Roche, Ipsen, Eisai, and Sanofi; institutional research funding: Pierre Fabre. The other authors have declared no conflicts of interest.
Figures
References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Powles T., Park S.H., Voog E. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. - PubMed
-
- Bajorin D.F., Dodd P.M., Mazumdar M. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173–3181. - PubMed
-
- Bellmunt J., Choueiri T.K., Fougeray R. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–1855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical