The efficacy and safety of itopride in feeding intolerance of critically ill patients receiving enteral nutrition: a randomized, double-blind study
- PMID: 33740892
- PMCID: PMC7976729
- DOI: 10.1186/s12876-021-01712-w
The efficacy and safety of itopride in feeding intolerance of critically ill patients receiving enteral nutrition: a randomized, double-blind study
Abstract
Background: Enteral feeding intolerance (EFI) is a frequent problem in the Intensive care unit (ICU) and is associated with poor clinical outcomes leading to worse prognosis in terms of mortality and ICU stay. Nowadays, prokinetic drugs are the mainstay of therapy in EFI. However, available prokinetics have uncertain efficacy and safety profiles. Itopride, is a prokinetic agent which is different and unique from the available prokinetics because of its dual mode of action as well as its tolerability and safety. The current study compared the efficacy and safety of Itopride against metoclopramide for EFI in critically ill patients. Moreover, it tested the utility and applicability of ultrasonography to measure gastric residual volume (GRV) in this population.
Methods: This randomized, double-blind study included 76 EFI patients who were randomly assigned to either Itopride or metoclopramide group. The primary outcome was to measure GRV by ultrasonography. Secondary outcomes included the percentage ratio of enteral feed volume, energy and protein received by patients over 7 days of treatment, ICU length of stay, safety parameters and occurrence of infectious complications or vomiting.
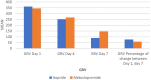
Results: Thirty-five patients of each group completed the study. At day 7, itopride significantly decreased GRV compared with metoclopramide group (p = 0.001). Moreover, there was a significant increase in the ratios of received enteral nutrition feed volume, calories, and protein after the one-week therapy in the itopride group more than the metoclopramide group (p = 0.001), (p = 0.002), (p = 0.01), respectively and there were no differences in any secondary outcomes or adverse events between the two groups.
Conclusion: In critically ill patients with EFI, itopride was well tolerated with superior efficacy to metoclopramide. In addition, we demonstrated that ultrasonography is a simple, non-invasive, inexpensive, and undemanding method for GRV measurements and can offer reliable assessments in the gastric emptying modality.
Trial registration: The trial was registered in ClinicalTrials.gov (NCT03698292). Date: October 5, 2018.
Keywords: Enteral feeding intolerance; Gastric residual volume; Itopride; Metoclopramide; Ultrasonography.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
A multicenter, randomized, double-blind study of ulimorelin and metoclopramide in the treatment of critically ill patients with enteral feeding intolerance: PROMOTE trial.Intensive Care Med. 2019 May;45(5):647-656. doi: 10.1007/s00134-019-05593-2. Epub 2019 May 6. Intensive Care Med. 2019. PMID: 31062046 Free PMC article. Clinical Trial.
-
Blinded, Double-Dummy, Parallel-Group, Phase 2a Randomized Clinical Trial to Evaluate the Efficacy and Safety of a Highly Selective 5-Hydroxytryptamine Type 4 Receptor Agonist in Critically Ill Patients With Enteral Feeding Intolerance.JPEN J Parenter Enteral Nutr. 2021 Jan;45(1):115-124. doi: 10.1002/jpen.1732. Epub 2020 Jan 28. JPEN J Parenter Enteral Nutr. 2021. PMID: 31990087 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Enteral Erythromycin Estolate in Combination With Intravenous Metoclopramide vs Intravenous Metoclopramide Monotherapy in Mechanically Ventilated Patients With Enteral Feeding Intolerance: A Randomized, Double-Blind, Controlled Pilot Study.JPEN J Parenter Enteral Nutr. 2021 Aug;45(6):1309-1318. doi: 10.1002/jpen.2013. Epub 2020 Oct 2. JPEN J Parenter Enteral Nutr. 2021. PMID: 32895971 Clinical Trial.
-
The efficacy and safety of prokinetics in critically ill adults receiving gastric feeding tubes: A systematic review and meta-analysis.PLoS One. 2021 Jan 11;16(1):e0245317. doi: 10.1371/journal.pone.0245317. eCollection 2021. PLoS One. 2021. PMID: 33428672 Free PMC article.
-
Gastric residual volume in critically ill patients: a dead marker or still alive?Nutr Clin Pract. 2015 Feb;30(1):59-71. doi: 10.1177/0884533614562841. Epub 2014 Dec 18. Nutr Clin Pract. 2015. PMID: 25524884 Review.
Cited by
-
Prediction of prokinetic agents in critically ill patients with feeding intolerance: a prospective observational clinical study.Front Nutr. 2023 Oct 27;10:1244517. doi: 10.3389/fnut.2023.1244517. eCollection 2023. Front Nutr. 2023. PMID: 37964927 Free PMC article.
-
Research progress on digestive disorders following traumatic brain injury.Front Immunol. 2024 Dec 20;15:1524495. doi: 10.3389/fimmu.2024.1524495. eCollection 2024. Front Immunol. 2024. PMID: 39759513 Free PMC article. Review.
-
Gastric ultrasound for feeding intolerance in the ICU: close but not quite right.BMC Gastroenterol. 2022 Jan 25;22(1):33. doi: 10.1186/s12876-022-02104-4. BMC Gastroenterol. 2022. PMID: 35078409 Free PMC article.
-
Feeding Intolerance-A Key Factor in the Management of Acute Pancreatitis: A Review.J Clin Med. 2024 Oct 24;13(21):6361. doi: 10.3390/jcm13216361. J Clin Med. 2024. PMID: 39518500 Free PMC article. Review.
-
Application of Touching Combined with Intelligent Interaction of Voice and Rhythm in Nursing Care of Newborns with Feeding Intolerance and Its Influence on Quality of Life.Comput Math Methods Med. 2022 Apr 1;2022:4747337. doi: 10.1155/2022/4747337. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jun 28;2023:9810782. doi: 10.1155/2023/9810782. PMID: 35401783 Free PMC article. Retracted. Clinical Trial.
References
-
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

