Tooth mousse containing casein phosphopeptide-amorphous calcium phosphate prevents biofilm formation of Streptococcus mutans
- PMID: 33740976
- PMCID: PMC7980609
- DOI: 10.1186/s12903-021-01502-6
Tooth mousse containing casein phosphopeptide-amorphous calcium phosphate prevents biofilm formation of Streptococcus mutans
Abstract
Background: Streptococcus mutans is a common cariogenic bacterium in the oral cavity involved in plaque formation. Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) has been introduced into tooth mousse to encourage remineralization of dental enamel. The aim of this research was to study the effect of tooth mousse containing CPP-ACP (GC Tooth Mousse®) or CPP-ACP with 0.2% fluoride (CPP-ACPF; GC Tooth Mousse Plus®; GCP) on S. mutans planktonic growth and biofilm formation.
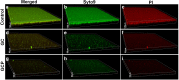
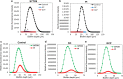
Methods: S. mutans was cultivated in the presence of different dilutions of the tooth mousse containing CPP-ACP or CPP-ACPF, and the planktonic growth was determined by ATP viability assay and counting colony-forming units (CFUs). The resulting biofilms were examined by crystal violet staining, MTT metabolic assay, confocal laser scanning microscopy (CLSM), and scanning electron microscope (SEM).
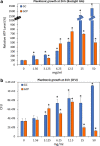
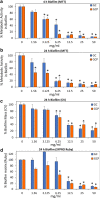
Results: The CPP-ACP tooth mousse (GC) at a dilution of 5-50 mg/ml (0.5-5%) did not inhibit planktonic growth, and even increased the ATP content and the number of viable bacteria after a 24 h incubation. The same was observed for the CPP-ACPF tooth mousse (GCP), except for the higher concentrations (25 and 50 mg/ml) that led to a drop in the bacterial count. Importantly, both compounds significantly decreased S. mutans biofilm formation at dilutions as low as 1.5-3 mg/ml. 12.5 mg/ml GC and 6.25 mg/ml GCP inhibited biofilm formation by 90% after 4 h. After 24 h, the MBIC90 was 6.25 mg/ml for both. CLSM images confirmed the strong inhibitory effect GC and GCP had on biofilm formation when using 5 mg/ml tooth mousse. SEM images of those bacteria that managed to form biofilm in the presence of 5 mg/ml tooth mousse, showed alterations in the bacterial morphology, where the streptococci appear 25-30% shorter on the average than the control bacteria.
Conclusion: Our data show that the tooth mousse containing CPP-ACP reduces biofilm formation of the cariogenic bacterium S. mutans without killing the bacteria. The use of natural substances which inhibit biofilm development without killing the bacteria, has therapeutic benefits, especially in orthodontic pediatric patients.
Keywords: Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP); Dental caries; GC Tooth Mousse®; Oral biofilm; Streptococcus mutans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

