Opioid therapy vs. multimodal analgesia in head and neck Cancer (OPTIMAL-HN): study protocol for a randomized clinical trial
- PMID: 33740977
- PMCID: PMC7980584
- DOI: 10.1186/s12904-021-00735-0
Opioid therapy vs. multimodal analgesia in head and neck Cancer (OPTIMAL-HN): study protocol for a randomized clinical trial
Abstract
Background: Radiation-induced mucositis (RIM) pain confers substantial morbidity for head and neck cancer (HNC) patients undergoing radiotherapy alone (RT) or chemoradiotherapy (CRT), often reducing treatment compliance. However, no standard currently exists for the treatment of RIM, and high dose opioid therapy, with its associated side effects and increased risk for chronic opioid use, remains the cornerstone of HNC pain management. The goal of this randomized clinical trial is to compare multimodal analgesia using analgesic medications with different mechanisms of action, to the institutional standard of opioid analgesia alone, in order to ascertain the optimal analgesic regimen for the management of RIM pain in HNC patients.
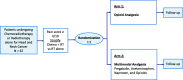
Methods: In this open-label, single-institution, non-inferiority, randomized clinical trial, sixty-two patients with mucosal head and neck malignancies treated with curative-intent radiation will be randomized in a 1:1 ratio, stratified by RT or CRT, between Arm 1: opioid analgesia alone as per the institutional standard, or Arm 2: multimodal analgesia using Pregabalin, Acetaminophen, and Naproxen, in addition to opioids, if required. The primary endpoint is the average 11-Numeric Rating Scale (11-NRS) score for pain during the last week of radiation treatment. Secondary endpoints include: average weekly opioid use, duration of opioid requirement, average daily 11-NRS score for pain, average weekly opioids dispensed, quality of life, hospitalizations for analgesic medication-induced complications, time to feeding tube insertion, weight loss, toxicity, treatment interruptions, and death within 3 months of completing RT treatment. Patients are eligible once analgesia is required for moderate 4/10 pain.
Discussion: This study will assess the efficacy and safety of multimodal analgesia and its impact on opioid requirements, clinical outcomes, and quality of life, as a potential new standard treatment for RIM pain in HNC patients undergoing definitive RT or CRT.
Trial registration: ClinicalTrials.gov Identifier: NCT04221165 . Date of registration: January 9, 2020. Appendix 2 reports the World Health Organization trial registration dataset.
Keywords: Head and neck Cancer; Multimodal analgesia; Non-inferiority; Opioid analgesia; Radiation Mucositis; Radiotherapy; Randomized clinical trial.
Conflict of interest statement
None to declare from the study sponsor/PIs or any study investigators.
Figures
References
-
- M V-L, G O, M H, S S. Oral Mucositis in Patients Undergoing Radiation Treatment for Head and Neck Carcinoma. Cancer. 2006;106(2). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials