A label-free, fast and high-specificity technique for plant cell wall imaging and composition analysis
- PMID: 33741013
- PMCID: PMC7980347
- DOI: 10.1186/s13007-021-00730-9
A label-free, fast and high-specificity technique for plant cell wall imaging and composition analysis
Abstract
Background: New cell wall imaging tools permit direct visualization of the molecular architecture of cell walls and provide detailed chemical information on wall polymers, which will aid efforts to use these polymers in multiple applications; however, detailed imaging and quantification of the native composition and architecture in the cell wall remains challenging.
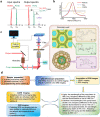
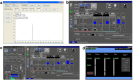
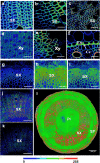
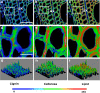
Results: Here, we describe a label-free imaging technology, coherent Raman scattering (CRS) microscopy, including coherent anti-Stokes Raman scattering (CARS) microscopy and stimulated Raman scattering (SRS) microscopy, which can be used to visualize the major structures and chemical composition of plant cell walls. We outline the major steps of the procedure, including sample preparation, setting the mapping parameters, analysis of spectral data, and image generation. Applying this rapid approach will help researchers understand the highly heterogeneous structures and organization of plant cell walls.
Conclusions: This method can potentially be incorporated into label-free microanalyses of plant cell wall chemical composition based on the in situ vibrations of molecules.
Keywords: Cell wall; Chemical composition; Coherent Raman scattering; Coherent anti-Stokes Raman scattering; Label-free imaging; Stimulated Raman scattering.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Grants and funding
- 31800504/National Natural Science Foundation of China
- 31761133009/National Natural Science Foundation of China
- 32030010/National Natural Science Foundation of China
- 111 project, B13007/the Program of Introducing Talents of Discipline to Universities
- 2018M631246/Postdoctoral Research Foundation of China
LinkOut - more resources
Full Text Sources
Other Literature Sources

