Chemogenetics drives paradigm change in the investigation of behavioral circuits and neural mechanisms underlying drug action
- PMID: 33741409
- PMCID: PMC8110310
- DOI: 10.1016/j.bbr.2021.113234
Chemogenetics drives paradigm change in the investigation of behavioral circuits and neural mechanisms underlying drug action
Abstract
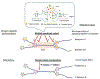
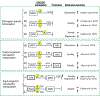
Recent developments in chemogenetic approaches to the investigation of brain function have ushered in a paradigm change in the strategy for drug and behavior research and clinical drug-based medications. As the nature of the drug action is based on humoral regulation, it is a challenge to identify the neuronal mechanisms responsible for the expression of certain targeted behavior induced by drug application. The development of chemogenetic approaches has allowed researchers to control neural activities in targeted neurons through a toolbox, including engineered G protein-coupled receptors or ligand-gated ion channels together with exogenously inert synthetic ligands. This review provides a brief overview of the chemogenetics toolbox with an emphasis on the DREADDs (Designer Receptors Exclusively Activated by Designer Drugs) technique used in rodent models, which is applicable to the investigation of how specific neural circuits regulate behavioral processes. The use of chemogenetics has had a significant impact on basic neuroscience for a better understanding of the relationships between brain activity and the expression of behaviors with cell- and circuit-specific orders. Furthermore, chemogenetics is potentially a useful tool to deconstruct the neuropathological mechanisms of mental diseases and its regulation by drug, and provide us with transformative therapeutics with medication. We also review recent findings in the use of chemogenetic techniques to uncover functional circuit connections of serotonergic neurons in rodent models.
Keywords: Animal models; Behavioral pharmacology; Chemogenetics; DREADDs; Drug treatment; Neural circuits; Research strategies.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures

References
-
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39. doi: 10.1016/j.neuron.2009.06.014. - DOI - PMC - PubMed
-
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, et al. (2011) Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. U.S.A 108, 18488–18493. doi: 10.1073/pnas.1104807108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

